Spinal cord injury (SCI) causes primary mechanical injury and secondary inflammation-mediated injury. Mechanical injury of spinal cord tissue causes primary injury, and secondary neuroinflammatory reaction occurs after primary injury, which mediates additional extensive nerve injury. Alleviating harmful acute neuritis can be used as a therapeutic strategy to inhibit injury and promote functional recovery. Microglia play an important role in secondary injury after spinal cord injury. In the acute phase of SCI, activated microglia can produce neurotoxic proinflammatory cytokines, such as IL-1β, TNF-α, and IL-6. Therefore, minimizing harmful effects of microglia and exerting its neuroprotective effects are very important to promote the recovery of nervous system.
Recently, researchers from Fujian Medical University published Inhibition of leucine rich repeats and calcium homology domain containing 1 accelerates microglia mediated neuroimaging in a rat traumatic spinal cord injury in The Journal of Neuroinflammation. In order to elucidate the significance of leucine-rich repeats and calponin homology domain containing 1 (LRCH1) on microglia function, researchers used lentivirus induced LRCH1 knockdown in primary microglia, and tested the role of LRCH1 in microglia mediated inflammation in vitro and in rat SCI model. It provides new clues for the study of novel treatments of SCI.

The expression of LRCH1 in microglia was down regulated after traumatic spinal cord injury. LRCH1 knockdown increases the generation of pro-inflammatory cytokines, such as IL-1 cytokines, TNF-α and IL-6. In addition, LRCH1 knockdown promoted microglia polarization to microglia with pro-inflammatory inducible nitric oxide synthase (iNOS) expression. LRCH1 knockdown also enhanced microglia mediated N27 neuronal death. Microglia infected by lentivirus carrying LRCH1 shRNA sequence is more toxic to primary rat spinal cord neurons
( purchased from Ubigene ).
Further study showed that LRCH1 knockdown increased the activation of p38 mitogen-activated protein kinase (MAPK) and Erk1/2 signaling, which are essential for the inflammatory response of microglia. When LRCH1 knockdown microglia were injected into the spinal cord of rats, they enhanced the production of pro-inflammatory cytokines, increased SCI induced leukocyte recruitment, aggravated SCI induced tissue injury and neuronal death, and worsened locomotor function.
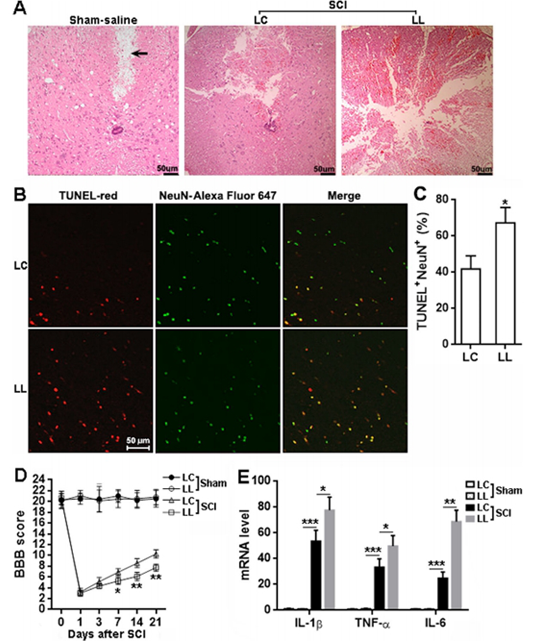
In conclusion, studies have shown that inhibition of LRCH1 can increase the production of pro-inflammatory cytokines by activated microglia. In addition, inhibition of LRCH1 promoted the polarization of microglia to pro-inflammatory status. The effect of LRCH1 is mediating Erk1/2 signaling by p38 MAPK reduction. The low expression of LRCH1 and the adoptive transfer of microglia aggravate the tissue injury and dysfunction caused by spinal cord injury. Therefore, inhibition of LRCH1 can accelerate microglial neuroinflammation after spinal cord injury. For the first time, LRCH1 serves as a negative regulator of microglia-mediated neuroinflammation after SCI and provides clues for developing novel therapeutic approaches against SCI.
Ubigene provides LRCH1 knockout or knockdown services.
The editing efficiency of CRISPR-U™ system, exclusively developed by Ubigene, is 10 times higher than that of traditional methods. We have successfully performed gene knockout in over 100 types of cell lines, including various brain and nervous system cell lines, such as Human Neuroblastoma Cell Line SK-N-SH, Rat Glioblastoma Cell Line C6, Mouse Hippocampal Neuron Cell Line HT22, etc. Contact us now to learn more about your research related services!



