Expert Insights | Practical Skills For
Gene-editing In HepG2 Cell

Today is the third session of Expert Insights for
HepG2. In this session, we will introduce some pratical skills for HepG2 cell culture and gene editing.
Let’s get started!
Hep G2 cell
line is a human liver cancer cell
line derived from liver tissue of a 15-year-old white male with hepatocellular carcinoma. This cell line
has extensive application value and can be used for establishing in vitro models of hepatoblastoma and liver
cancer, studying liver drug metabolism pathways, and constructing models of hepatitis virus
infection, etc. In addition, gene-editing Hep G2 cell line is also an important tool in the study of
the pathogenesis of liver cancer, drug
resistance mechanisms, and cancer treatment.
However, to ensure the stability of Hep G2 cells and the reliability of
experimental results, it is particularly important to master good cell culture techniques. Let's learn together
how to culture Hep G2 cells properly.
First, let's learn some basic information about Hep G2.
Cell Name
|
Hep G2 (human liver cancer cell)
|
Growth Characteristics
| YC-C001 |
Cell Morphology
|
Epithelial-like, adherent growth; forming
sheet-like islands, easy to clump, cluster and stack
|
Cell Culture Medium
| DMEM+10%FBS+1%P/S; |
Culture Environment
|
Air,95%;CO2,5%;37℃
|
Medium Change Frequency
| 2~3days/time |
Passage Ratio
| 1:2-1:4 |
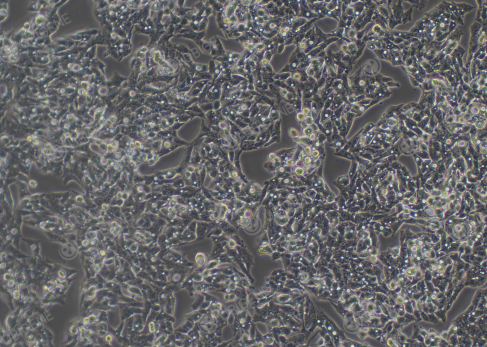
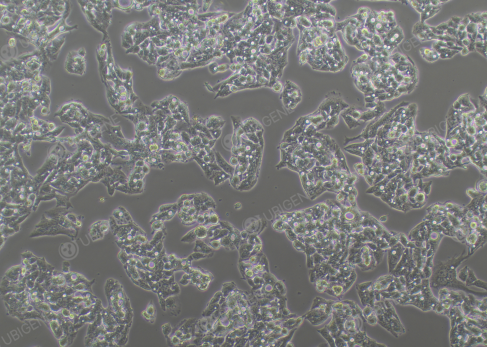
Figure 1. Hep G2 Cell Image (Normal Growth) (Left: High confluency; Right: Low
confluency)
Cell Thawing
1) Preparation: warm up the complete culture medium in 37°C water bath;
2) Inside the ultra-clean bench, pipet 7 ml of pre-warmed complete
medium into a 15 ml centrifuge tube;
3) Take out the cryopreserved vial from dry ice, hold the cap with forceps, quickly thaw cells in a
37°C water bath by gently swirling the vial (Note: keep the cap out of the water) for 1 minute to completely
thaw the cells;
4) Transfer the thawed cells to the prepared centrifuge tube with complete
medium (from step 2), close the lid, and centrifuge at 1100 rpm for 4 mins at
room temp to collect the cells;
5) Meanwhile, prepare a new T25 flask, add 4mL complete culture medium;
6) After centrifugation, carefully remove and discard the supernatant. Resuspend
cell pellet with 1ml of complete medium and then transfer to the flask (from step
5), incubate the flask in a incubator.
7) Observe the cell status and adhesion the next day.
Cell Passaging Procedure (using
T25)
1) When the confluence reaches 80-90%, it is ready to passage. Remove the old
culture medium, add 5mL PBS and rinse the cells 1-2 times;
2) Add 1mL trypsin, gently shake the flask to allow trypsin completely cover the
cells, place the flask into the incubator and incubate for 1-3 mins, until the majority of the cells
become round and non-adherent as observed under the microscope, a large number of cells detached from
each side when gently shaking and tapping the flask, terminate trypsin digestion immediately;
3) Add complete medium to stop digestion, the volume is 2 times of trypsin. Then transfer the cell suspension to a 15mL centrifuge tube;
4) Centrifuge at 1100 rpm for 4 mins at room temp. After centrifugation, remove and
discard the supernatant and resuspend the cells with complete medium
5) Passage the cells at a ratio of 1:2-1:4,
and observe the cell status the next day.
Notes:
1. Hep G2 cells grow in clumps. Cell passaging must be a complete passaing, tap the flask gently
until the cell clumps completely detach before stopping digestion to avoid clumping.
2. When resuspending the
cells by pipetting, try to make the cells into single
cells, but be careful to pipet gently to avoid causing damage to the cells.
3. Cells are sensitive to culture conditions, especially to serum, so it
is necessary to choose high-quality fetal bovine serum for culture.
4. Due to clump growth characteristics, the cell morphology after thawing may differ from normal cell morphology, which is
normal. After several passages, the cell morphology will return to normal.
5. A few air bubbles may appear during cell culture, which is normal.
Cell Cryopreservation:
1) Same as procedures of cell passaging, digest the cells to a single-cell
suspension, and transfer to a centrifuge tube;
2) Mix well by pipetting and take 20μL for cell counting;
3) Centrifuge at 1100 rpm for 4 mins at
room temp. After centrifugation, remove and discard the supernatant, and resuspend the cells with 1-2mL of 4℃ pre-cooled cryopreservation medium, then add cryopreservation medium to adjust to the required density
(1*10^6 cells/mL);
4) Aliquot the cell suspension to
cryovials as 1 ml/tube;
5) Place the cryovials in 4℃
pre-cooled Freezing Container, then put the container in an ultra-low temperature freezer;
6) Stay overnight, transfer the cryovials
to liquid nitrogen for long-term storage.
FAQs
How to avoid low cell thawing viability?
1. Thawing process should be fast and steady.
2. Use correct culture conditions: culture medium, serum, temperature, CO2
concentration, etc., avoid over-vibration of the culture flask, reduce mechanical damage to the
cells.
3. When thawing, it may be advisable to culture the cells in a small culture plate
first, and expand after the small plate is full.
4. Make sure the cells are in a good growth state before cryopreservation.
5. Before freezing, the cells can be rinsed with PBS twice after the digestion is
stopped.
6. Consider increasing the freezing density appropriately.
How to adjust poor cell status?
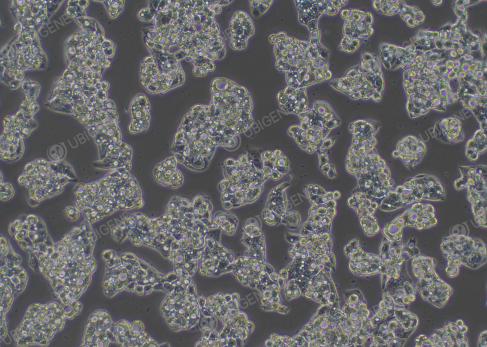
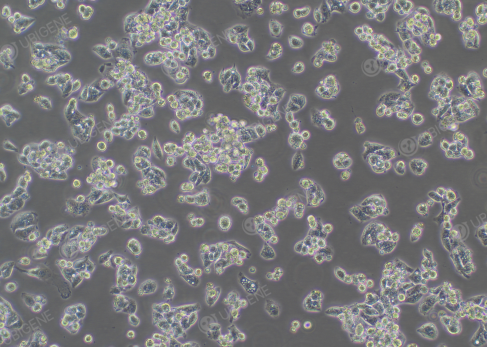
Figure 2. Hep G2 in poor cell status
1. Culture medium and serum: ensure the correct
use of basal culture medium and adequate serum; Hep G2 cells can be cultured with increased
serum concentration.
2. Cell culture environment: confirm if the
culture temperature, humidity, and gas conditions are normal.
3. Cell passaging and
medium change operations: when passaging the cells, pay attention to
the digestion time and trypsin concentration to avoid cell damage caused by too long or too short digestion
times; perform medium change every 2-3 days to avoid prolonged lack of
medium change.
4. Cell thawing and cryopreservation operations: Ensure standardized cell
thawing and cryopreservation operations to reduce cell damage and death.
5. If cell density is low, consider increasing cell
density to above 70%; high cell density increases the secretion of cytokines,
which is beneficial for cell proliferation.
How to deal with dead cells on the cell
surface?
1. Shake with PBS twice for washing, then add trypsin
for rinsing; when there are signs of dead cells about to lift off the surface,
quickly discard the trypsin.
2. Wash the cells again with PBS, then add trypsin for
normal digestion.
3. After stopping digestion and centrifuging, add
PBS to resuspend and wash the cells again.
Gene Editing Tips
How to improve transfection efficiency in Hep G2
cells?
1. Ensure that the cells are in a good state, in the logarithmic
growth phase, and with moderate cell density.
2. Be careful
to the digestion time of the cells, avoid over-digestion that might damage the
cells.
3. Experiment with a cell viability greater than 80%.
4. During experiments, try to make the cells into single cells to prevent
cell clumping.
5. Different transfection methods have different details to consider;
commonly used methods include electroporation and lentivirus transduction:
Electroporation
| Lentivirus
transduction
|
1.Control the cell amount for
electroporation 2.Ensure that the cell
adhesion rate after electroporation is ≥ 50% 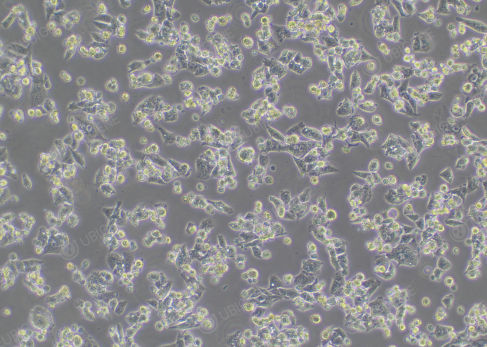 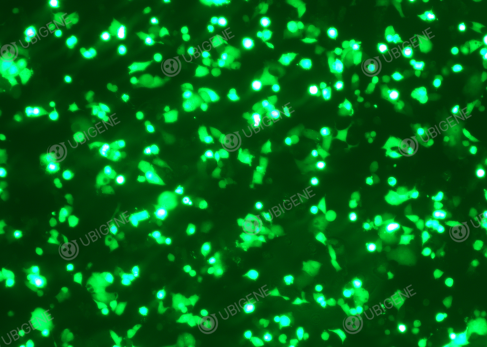
Figure 3.
Electroporated HepG2 cells
| 1.Control the cell
confluency before transduction, it should not be too
high
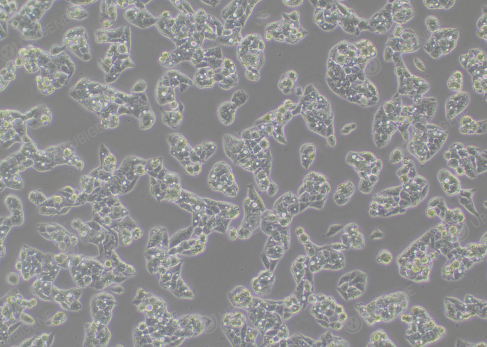 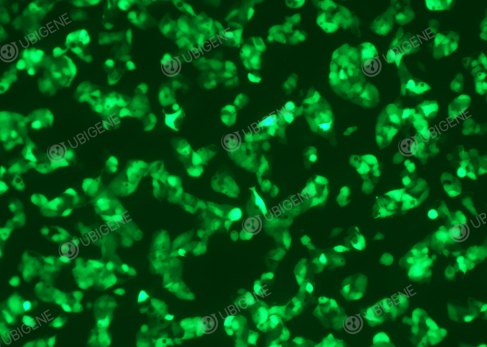
Figure 4.
Lentivirus transduced HepG2 cells
|
How to increase the clonal formation rate of Hep G2
cells?
1. The viability of cells during single-cell cloning needs to
be >80%
2. Use high-quality fetal bovine serum during cloning
3. Wash with PBS once during
cloning
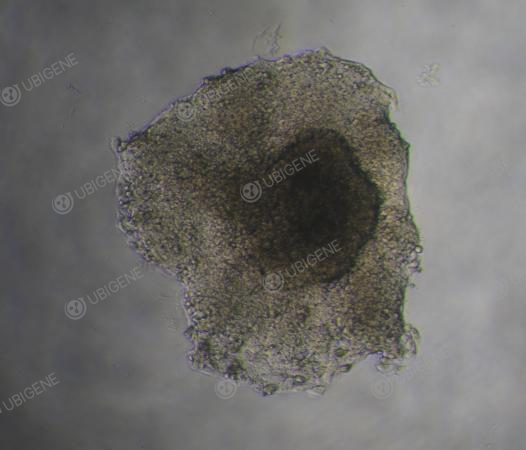
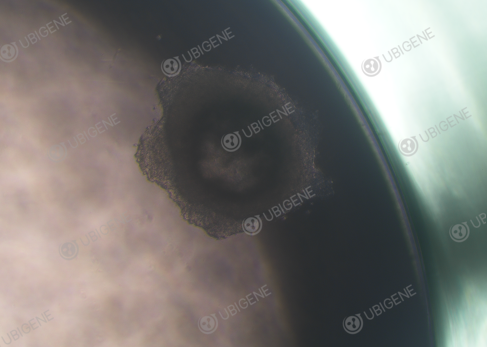
Figure 5. Monoclonal HepG2
It's been another day full of valuable information. We
hope these tips on culturing and transfecting Hep G2 cells will help your experiment!
Ubigene’s Hep G2 cell line, selected from hundreds
of gene editing experiments, is available for sales! And we also provide gene editing services. If you
want to easily obtain high-quality Hep G2 cells, feel free to contact us. We currently offer 300+
popular gene KO cell lines as low as $1780 each (including in the field of liver cancer)~
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project