Published on: October 15, 2024
Views:
--
Expert Insights | Jurkat Cell Culture and Gene Editing Tips Are Here!

Jurkat cells are a type of suspension cell, established by Schneider, originating from the peripheral blood of a 14-year-old boy. Jurkat, Clone E6-1 is a clone of the Jurkat-FHCRC cell line (a Jurkat derivative), which can produce large amounts of IL-2 upon induction. Additionally, this cell line can express a series of typical T lymphocyte surface markers and secrete various cytokines, making it an important model cell for research in T cell immune function, leukemia, viral infection, and signal transduction.
However, during the culture of Jurkat cells, some issues may arise, such as increased cell debris and excessive cell clumping, which can affect the accuracy of experimental results and the stability of cell conditions. Today, let's follow us to learn about the tips for Jurkat cell culture and gene editing!
Cell Information:
Cell Name | Human Leukemia T Lymphocyte(Jurkat, Clone E6-1) |
Growth Properties | Grow in suspension, it will come with a cell clustering phenomenon |
Cell Morphology | Lymphocyte-like,suspension |
Cell Culture Medium Culture Environment | 90%RPMI-1640+10%FBS Air, 95%; CO2, 5%; 37°C |
Medium Change Frequency | Change medium when passaging |
Passage Ratio | 1:3-1:5 |
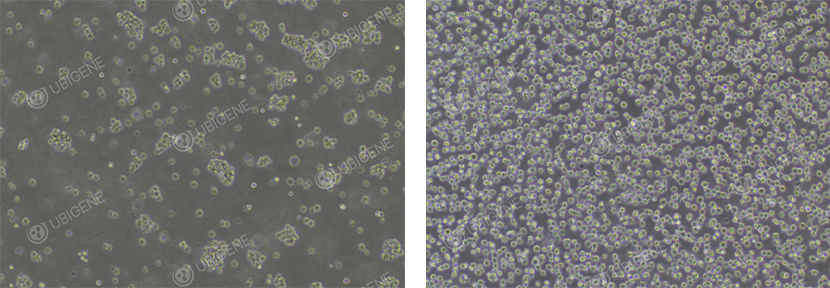
Figure 1. Jurkat Cell Image (Normal Growth)
Jurkat Cell Culture
Cell Thawing:
1) Preparation: warm up the complete culture medium in 37°C water bath. Get the cells ready;
2) Inside the ultra-clean bench, pipet 7 mL of complete medium into a 15 mL centrifuge tube;
3) Take out the cryopreserved vial from dry ice, hold the cap with forceps, quickly thaw cells in a 37°C water bath by gently swirling the vial (Note: keep the cap out of the water). In about 1 minute, it would completely thaw;
4) Transfer the thawed cells to the prepared centrifuge tube (step 2) by pipette, close the lid, and centrifuge at 1100 rpm for 4 mins at room temp to collect the cells;
5) Inside the ultra-clean bench, carefully remove and discard the supernatant. Resuspend cell pellet with 1mL of fresh complete medium and then transfer to a T25 flask (or 6 cm culture dish) containing 4 mL of complete medium, label the flask with cell name, date and passage no., incubate the flask in a 37℃, 5%CO2 incubator;
6) Observe the cell condition in the next day.
Cell Passaging:
Centrifugation method:
1) Transfer all the cell suspension to a centrifuge tube and centrifuge at 1100 rpm for 4 minutes;
2) After centrifugation, remove and discard the supernatant and resuspend the cells with appropriate amount of complete medium by pipette, and take 20 ul of cells for cell counting;
3) According to the cell counting results, adjust the cell density to 2x105~4.0x105cells/mL, and culture the cells in different sizes of culture flasks depending on the cell density;
4) Incubate the flask in a 37℃, 5%CO2 incubator.
Half medium replacement method (recommended):
1) Add fresh complete medium to the flask, gently pipet the cells in the culture flask evenly;
2) Divide the cell suspension evenly into new culture flasks according to the appropriate passage ratio, and add an appropriate amount of fresh complete culture medium to each flask.
3) Incubate the flask in a 37℃, 5%CO2 incubator.
Cell cryopreservation:
1) Transfer all the cell suspension to a centrifuge tube
2) Centrifuge at 1100 rpm for 4 minutes at room temp; After centrifugation, remove and discard the supernatant, and resuspend the cells with appropriate amount of 4℃ pre-cooled cryopreservation medium, take 20 μL for cell counting, then add cryopreservation medium to adjust to the required density (5×106-1x107cells/mL);
3) Aliquot the cell suspension to cryovials as 1 mL/tube, place the cryovials in 4°C pre-cooled Freezing Container, then put the container in -80℃ freezers;
4) Stay overnight, transfer the cryovials to liquid nitrogen for long-term storage.
Note:
1. It is recommended that the cell viability be greater than 90% during cryopreservation, and the amount of cells per vial should be controlled at 5*10^6-1*10^7/ml
2. Avoid frequent centrifugation and medium change during cell culture. It is generally recommended to use the half medium replacement method for passaging when the cells are in good condition
3. The cell is sensitive to mechanical forces. During normal culture process, cells can be gently pipetted to avoid excessive pipetting force that may cause cell differentiation and cell death
4. Control the cell density during culture, and keep the cells within an appropriate density range during culture and passaging
Gene Editing Tips
1.Too many cell debris during cell culture or dead cells after antibiotic screening:
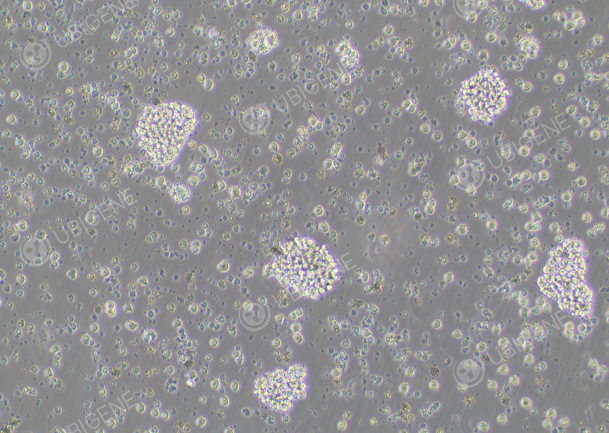
Figure 2. Many cell debris and dead cells observed
If there are too many cell debris or dead cells, after centrifugation, you can add an appropriate amount of PBS to resuspend the cells, transfer the cell suspension to a 1.5 ml EP tube for low-speed centrifugation (800 rpm for 3 minutes), carefully remove PBS after centrifugation. This step can be repeated for two times based on the amount of cells, then seed in a suitable culture plate (preferably a small plate)
2.Cell clumps occur during cell culture:
A small amount of cell clustering is normal, but if there are too many clumps, consider the following solutions:
1) Jurkat, Clone E6-1 cells have certain requirements for the quality of serum, try switching to a better serum brand
2) Control the cell density well, not too sparse or too dense, keep the cell density in suitable range
3.Low viability after cryopreservation and thawing (low survival rate):
Because Jurkat, Clone E6-1 cells are suspension cells, they are sensitive to cryopreservation, so it is recommended to increase the cell density during cryopreservation to improve the survival rate of thawed cells, it is recommended to have a cell density of at least 5*10^6-1*10^7 during cryopreservation.
You can thaw the cells in a well of a 6-well plate, transfer them to a larger plate after the cell condition has recovered.
4.How to improve monoclonal formation rate:
1) Cell viability needs to be >90% when isolating clones
2) The cell counting result after dilution is best between 1×106-2×106
3) It is recommended to use premium fetal bovine serum
4) When isolating clones, it is recommended to wash with PBS twice
5.What to notice during cell transfection?
1) Cell viability > 90%, cells should be in good condition, visually they are round, transparent, and have intact cell membranes
2) During the transfection process, it is important to have gentle operation
3) For viral infection method, it is recommended to conduct a pre-experiment to find the optimal MOI and antibiotic screening concentration before formal experiments; ensuring that the titer of the infecting virus meets standards; add the transfection-enhanced agent Polybrene before infection; infection is best performed in a 24-well plate or 12-well plate
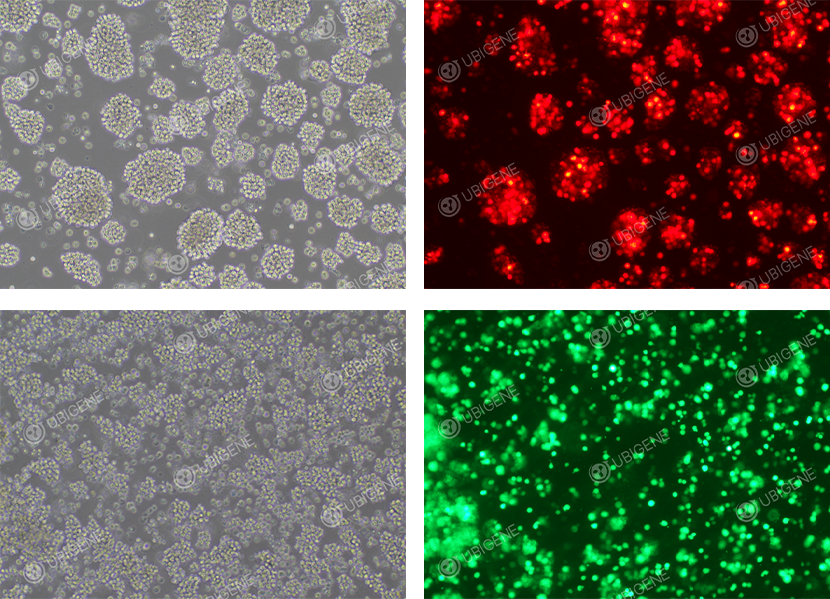
Figure 3. Post-electroporated Jurkat Cell Line
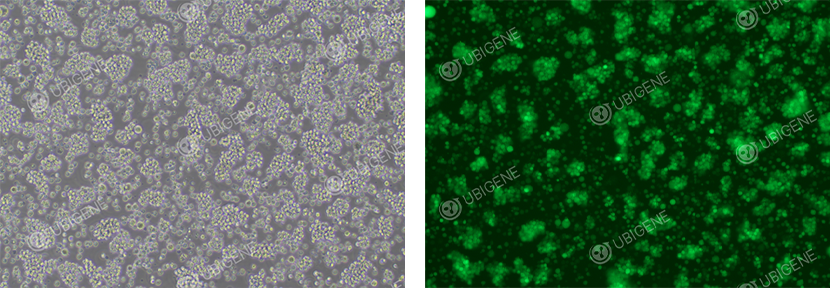
Figure 4. Post-infected (Lentivirus) Jurkat Cell Line
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project