Location:Home > Application > IF=14.9 | Ubigene’s Lentivirus assisted to get new breakthroughs in HNSCC study

Head and Neck Squamous Cell Carcinoma (HNSCC) refers to squamous cell carcinoma that occurs in multiple anatomical sites of the head and neck. It has a high incidence and poor prognosis in the middle and late stages. Its carcinogenic factors include smoking, alcohol dependence, HPV infection, etc. Cancer stem cells (CSCs) play an indispensable role in the heterogeneity, metastasis and treatment resistance of HNSCC.
Recently, researchers from Wuhan University published an article entitled “LIMP-2 enhances cancer stem-like cell properties by promoting autophagy-induced GSK3β degradation in head and neck squamous cell carcinoma” on International Journal of Oral Science (IF=14.9), of which Ubigene’s lentivirus with a high titer, high viability, and stable expression, was used and combined with a large number of experiments to help identify LIMP-2 gene as a potential regulator of HNSCC progression. This article comprehensively described the mechanism of LIMP-2 promoting HNSCC cancer progression and revealed the connection between autophagy, CSCs, and immunotherapy resistance.
If you have experimental needs of lentiviruses, or want to free your hands and time from stable cell line construction, contact us to outsource your experiment >>>
Researchers first screened LIMP-2 as a potential regulator of HNSCC based on the bioinformatics analysis of autophagy-lysosome pathway. The expression of LIMP-2 was higher in HNSCC tissues, and it was observed that LIMP-2 was significantly upregulated in 23 types of cancers. The expression of LIMP-2 in HNSCC tissue microarray (TMA) was studied by immunohistochemical (IHC) staining and it was found that the expression of LIMP-2 was significantly increased, and was positively correlated with pathological grade, lymph node metastasis and tumor size. Moreover, its expression is higher in women. It was also found that the expression of LIMP-2 was an independent risk factor for the overall survival (OS) of HNSCC patients (Figure 1). In a variety of cancers, LIMP-2 is generally positively correlated with autophagy related genes.
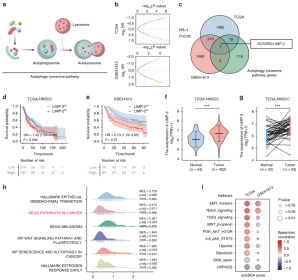
Figure 1. Bioinformatics analysis showed that LIMP-2 is a potential regulator of HNSCC
Subsequently, researchers constructed three stable overexpression / knockdown mouse cell lines, and helped determine the potential role of LIMP-2 in regulating autophagy and CSC.
EZ-editor™ Series Product - Lentiviral Packaging Kit uses a third-generation packaging system with Ubigene exclusive developed formula and it is easy to use, improving the efficiency of your gene-editing experiment.
In order to study the specific mechanism of LIMP-2 regulating autophagy, researchers detected the expression of autophagy marker LC3B-II and autophagy substrate p62/ SQSTM1. In LIMP-2 knockdown 4MOSC2 cells, the expression of LC3B-II and p62 was significantly upregulated, while in 4MOSC2 cells with LIMP-2 overexpression, the expression of LC3B-II and p62 was decreased. However, compared with the negative control group, the expression levels of ATG5 and ATG7 which are the key molecules involved in autophagosome formation were not significantly changed. While through microscopy, the increased accumulation of autophagosomes in cancer cells after LIMP-2 knockdown was observed. Therefore, researchers speculated that this phenomenon may be due to the inhibition of autophagic flux. To further verify whether autophagic flux was blocked, mRFP-GFP-LC3 plasmid was transfected to detect autophagic flux. In the LIMP-2 knockdown group, the ratio of autophagolysosomes and autophagosomes was significantly lower than that in the control group, indicating that autophagic flux was impaired after LIMP-2 knockdown. In contrast, in 4MOSC1 cells, ectopic LIMP-2 expression increased the ratio of autophagolysosomes to autophagosomes.
Then, researchers explored the effect of LIMP-2 on the tumorigenicity of HNSCC. Compared with the control, the viability and clone formation ability of the LIMP-2 knockdown HNSCC cells were significantly reduced, and the number of cells was significantly downregulated. In 4MOSC2 and SCC7 allograft models, LIMP-2 knockdown significantly inhibited tumor growth (Figure 2).
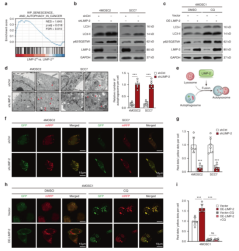
Figure 2. LIMP-2 promoted autophagy in HNSCC by participating in the formation of autophagosomes
Combining the results of in vitro and in vivo experiments with the analysis of patient specimens, it showed that LIMP-2 could promote autophagy and stem cell properties of HNSCC. Targeting autophagy can effectively inhibit the invasiveness of CSCs in HNSCC. LIMP-2 overexpression increased the expression of CSCs related markers, which were significantly inhibited by chloroquine (CQ) in 4MOSC1 cells, confirming that CQ inhibited the tumor formation ability of the LIMP-2 overexpressing MOSC1 cells. These results suggested that autophagy is involved in the malignant phenotype of HNSCC driven by LIMP-2.
Next, researchers further knocked down β-catenin in the the LIMP-2 overexpressing MOSC1 cells and it significantly inhibited the enhanced expression of CSCs-related markers induced by LIMP-2 overexpression. Similar results were also observed in colony formation and tumorsphere formation experiments. In 4MOSC2 orthotopic HNSCC mouse model, knockdown of LIMP-2 resulted in reduced expression of β-catenin and increased expression of GSK3β, and the samples of patients with low expression of LIMP-2 tended to have lower β-catenin expression and higher GSK3β expression (Figure 3). In conclusion, LIMP-2 activates GSK3β/β-catenin signaling pathway to regulate the stemness potential of HNSCC cells.

Figure 3. LIMP-2 exerts oncogenic effects by activating the GSK3β/β-catenin pathway
In the case of Wnt/β-catenin signaling activation, GSK3β, GSK3β is sequestered in the lumen of late endosomes and degraded by lysosomes. Recent findings suggest that autophagy is involved in the degradation of GSK3β to maintain the high expression of β-catenin, suggesting that autophagy might be related to Wnt signaling pathway. Considering the functional role of LIMP-2 in the autophagy lysosome pathway, researchers concluded that LIMP-2 regulates GSK3β degradation. Cycloheximide (CHX) experiments showed that in the case of LIMP-2 knockdown, GSK3β degradation was delayed. In contrast, overexpression of LIMP-2 significantly reduced the half-life of GSK3β. These results indicated that LIMP-2 accelerates GSK3β degradation.
Recent studies showed that targeting CSCs can improve the effectiveness of immunotherapy. In order to further evaluate the impact of LIMP-2 on the potential efficacy of immunotherapy, researchers used TIDE algorithm to evaluate TCGA database. The TIDE score of the low expression group of LIMP-2 was significantly lower than that of the high expression subgroup of LIMP-2, which indicated that patients with low expression of LIMP-2 were more likely to benefit from immune checkpoint inhibitor (ICI) treatment than patients with high expression of LIMP-2. The 4MOSC2 model was further used to test the potential combination efficacy of LIMP-2 knockdown with αPD-1 therapy and it was found that after αPD-1 treatment, the tumor growth of LIMP-2 knockdown cell line was significantly inhibited. These findings suggested that targeting LIMP-2 can overcome αPD-1 treatment resistance, improving the effect of immunotherapy (Figure 4). Therefore, LIMP-2 may be a reliable biomarker to predict the response of patients to PD-1 blockade therapy, which can provide precise guidance for clinical intervention.

Figure 4. Knockdown of LIMP-2 enhances immunotherapy response
The treatment of HNSCC still faces the challenge of high recurrence rate and high mortality, which is closely related to cancer stem cells. Researchers found a new role of LIMP-2 in promoting the formation of HNSCC stem cells. LIMP-2 degrades GSK3β via autophagy to promote the CSC properties of HNSCC cells. LIMP-2 is an independent poor prognostic factor for HNSCC. The elevated expression of LIMP-2 is beneficial to maintain the CSC phenotype, which means that the immunotherapy resistance of HNSCC increases. This study revealed convincing evidence demonstrating the potential key role of LIMP-2 as a diagnosis and treatment of HNSCC.



Head and Neck Squamous Cell Carcinoma (HNSCC) refers to squamous cell carcinoma that occurs in multiple anatomical sites of the head and neck. It has a high incidence and poor prognosis in the middle and late stages. Its carcinogenic factors include smoking, alcohol dependence, HPV infection, etc. Cancer stem cells (CSCs) play an indispensable role in the heterogeneity, metastasis and treatment resistance of HNSCC.
Recently, researchers from Wuhan University published an article entitled “LIMP-2 enhances cancer stem-like cell properties by promoting autophagy-induced GSK3β degradation in head and neck squamous cell carcinoma” on International Journal of Oral Science (IF=14.9), of which Ubigene’s lentivirus with a high titer, high viability, and stable expression, was used and combined with a large number of experiments to help identify LIMP-2 gene as a potential regulator of HNSCC progression. This article comprehensively described the mechanism of LIMP-2 promoting HNSCC cancer progression and revealed the connection between autophagy, CSCs, and immunotherapy resistance.
If you have experimental needs of lentiviruses, or want to free your hands and time from stable cell line construction, contact us to outsource your experiment >>>
Researchers first screened LIMP-2 as a potential regulator of HNSCC based on the bioinformatics analysis of autophagy-lysosome pathway. The expression of LIMP-2 was higher in HNSCC tissues, and it was observed that LIMP-2 was significantly upregulated in 23 types of cancers. The expression of LIMP-2 in HNSCC tissue microarray (TMA) was studied by immunohistochemical (IHC) staining and it was found that the expression of LIMP-2 was significantly increased, and was positively correlated with pathological grade, lymph node metastasis and tumor size. Moreover, its expression is higher in women. It was also found that the expression of LIMP-2 was an independent risk factor for the overall survival (OS) of HNSCC patients (Figure 1). In a variety of cancers, LIMP-2 is generally positively correlated with autophagy related genes.

Figure 1. Bioinformatics analysis showed that LIMP-2 is a potential regulator of HNSCC
Subsequently, researchers constructed three stable overexpression / knockdown mouse cell lines, and helped determine the potential role of LIMP-2 in regulating autophagy and CSC.
EZ-editor™ Series Product - Lentiviral Packaging Kit uses a third-generation packaging system with Ubigene exclusive developed formula and it is easy to use, improving the efficiency of your gene-editing experiment.
In order to study the specific mechanism of LIMP-2 regulating autophagy, researchers detected the expression of autophagy marker LC3B-II and autophagy substrate p62/ SQSTM1. In LIMP-2 knockdown 4MOSC2 cells, the expression of LC3B-II and p62 was significantly upregulated, while in 4MOSC2 cells with LIMP-2 overexpression, the expression of LC3B-II and p62 was decreased. However, compared with the negative control group, the expression levels of ATG5 and ATG7 which are the key molecules involved in autophagosome formation were not significantly changed. While through microscopy, the increased accumulation of autophagosomes in cancer cells after LIMP-2 knockdown was observed. Therefore, researchers speculated that this phenomenon may be due to the inhibition of autophagic flux. To further verify whether autophagic flux was blocked, mRFP-GFP-LC3 plasmid was transfected to detect autophagic flux. In the LIMP-2 knockdown group, the ratio of autophagolysosomes and autophagosomes was significantly lower than that in the control group, indicating that autophagic flux was impaired after LIMP-2 knockdown. In contrast, in 4MOSC1 cells, ectopic LIMP-2 expression increased the ratio of autophagolysosomes to autophagosomes.
Then, researchers explored the effect of LIMP-2 on the tumorigenicity of HNSCC. Compared with the control, the viability and clone formation ability of the LIMP-2 knockdown HNSCC cells were significantly reduced, and the number of cells was significantly downregulated. In 4MOSC2 and SCC7 allograft models, LIMP-2 knockdown significantly inhibited tumor growth (Figure 2).

Figure 2. LIMP-2 promoted autophagy in HNSCC by participating in the formation of autophagosomes
Combining the results of in vitro and in vivo experiments with the analysis of patient specimens, it showed that LIMP-2 could promote autophagy and stem cell properties of HNSCC. Targeting autophagy can effectively inhibit the invasiveness of CSCs in HNSCC. LIMP-2 overexpression increased the expression of CSCs related markers, which were significantly inhibited by chloroquine (CQ) in 4MOSC1 cells, confirming that CQ inhibited the tumor formation ability of the LIMP-2 overexpressing MOSC1 cells. These results suggested that autophagy is involved in the malignant phenotype of HNSCC driven by LIMP-2.
Next, researchers further knocked down β-catenin in the the LIMP-2 overexpressing MOSC1 cells and it significantly inhibited the enhanced expression of CSCs-related markers induced by LIMP-2 overexpression. Similar results were also observed in colony formation and tumorsphere formation experiments. In 4MOSC2 orthotopic HNSCC mouse model, knockdown of LIMP-2 resulted in reduced expression of β-catenin and increased expression of GSK3β, and the samples of patients with low expression of LIMP-2 tended to have lower β-catenin expression and higher GSK3β expression (Figure 3). In conclusion, LIMP-2 activates GSK3β/β-catenin signaling pathway to regulate the stemness potential of HNSCC cells.

Figure 3. LIMP-2 exerts oncogenic effects by activating the GSK3β/β-catenin pathway
In the case of Wnt/β-catenin signaling activation, GSK3β, GSK3β is sequestered in the lumen of late endosomes and degraded by lysosomes. Recent findings suggest that autophagy is involved in the degradation of GSK3β to maintain the high expression of β-catenin, suggesting that autophagy might be related to Wnt signaling pathway. Considering the functional role of LIMP-2 in the autophagy lysosome pathway, researchers concluded that LIMP-2 regulates GSK3β degradation. Cycloheximide (CHX) experiments showed that in the case of LIMP-2 knockdown, GSK3β degradation was delayed. In contrast, overexpression of LIMP-2 significantly reduced the half-life of GSK3β. These results indicated that LIMP-2 accelerates GSK3β degradation.
Recent studies showed that targeting CSCs can improve the effectiveness of immunotherapy. In order to further evaluate the impact of LIMP-2 on the potential efficacy of immunotherapy, researchers used TIDE algorithm to evaluate TCGA database. The TIDE score of the low expression group of LIMP-2 was significantly lower than that of the high expression subgroup of LIMP-2, which indicated that patients with low expression of LIMP-2 were more likely to benefit from immune checkpoint inhibitor (ICI) treatment than patients with high expression of LIMP-2. The 4MOSC2 model was further used to test the potential combination efficacy of LIMP-2 knockdown with αPD-1 therapy and it was found that after αPD-1 treatment, the tumor growth of LIMP-2 knockdown cell line was significantly inhibited. These findings suggested that targeting LIMP-2 can overcome αPD-1 treatment resistance, improving the effect of immunotherapy (Figure 4). Therefore, LIMP-2 may be a reliable biomarker to predict the response of patients to PD-1 blockade therapy, which can provide precise guidance for clinical intervention.

Figure 4. Knockdown of LIMP-2 enhances immunotherapy response
The treatment of HNSCC still faces the challenge of high recurrence rate and high mortality, which is closely related to cancer stem cells. Researchers found a new role of LIMP-2 in promoting the formation of HNSCC stem cells. LIMP-2 degrades GSK3β via autophagy to promote the CSC properties of HNSCC cells. LIMP-2 is an independent poor prognostic factor for HNSCC. The elevated expression of LIMP-2 is beneficial to maintain the CSC phenotype, which means that the immunotherapy resistance of HNSCC increases. This study revealed convincing evidence demonstrating the potential key role of LIMP-2 as a diagnosis and treatment of HNSCC.


