Location:Home > Application > Ubigene’s KO cells assist in revealing genomic landscape and evolutionary history of HCC in Chinese individuals
Nature|Ubigene's KO cells assist in revealing genomic landscape and evolutionary history of HCC in Chinese individuals

Abstract
On February 14, 2024, the research group led by Hongyang Wang from National Center for Liver Cancer/Eastern Hepatobiliary Surgery Hospital, Shanghai, China and the Biomedical Pioneering Innovation Center (BIOPIC), Beijing Advanced Innovation Center for Genomics (ICG), School of Life Sciences, Peking University, Beijing, China jointly published the latest research results entitled Deep whole-genome analysis of 494 hepatocellular carcinomas on Nature, and completed the Chinese Liver Cancer Atlas (CLCA). In this study, 494 HCC patients' tumor tissues from different regions of China were subjected to high-depth whole genome sequencing (average depth, 120x), and the coding and non-coding driver genes, mutational signatures, copy number variations, clustered alternation: chromothripsis, chromoplexy and kataegis, extrachromosomal circular DNA (ecDNA), and the characteristics of mutation evolution were analyzed in depth. Three newly identified potential driver events were also selected for detailed functional verification. Based on CRISPR site directed mutagenesis and knockdown / knockout experiments in multiple cell lines, it was found that mutations in the above genes were sufficient to cause significant changes in gene expression levels (Among them, PPP1R12B, KCNJ12, FGA knockout cells and overexpression lentiviruses carrying mutations were all constructed by Ubigene), and were involved in regulating various malignant phenotypes of hepatocellular carcinoma, These results confirm the validity of the new driving events found based on data analysis.
Background
The mortality of primary liver cancer ranks third among all cancers in the world. Hepatocellular carcinoma (HCC) is its main subtype, accounting for more than 80% of all cases. More than half of the new cases of liver cancer in the world occur in China every year, and the prognosis of patients is generally poor. The five-year overall survival rate of patients with hepatocellular carcinoma in China is only about 10%. The etiology of hepatocellular carcinoma is complex, among which HCV infection is the most common in the Japanese population. Alcohol, non-alcoholic steatohepatitis and metabolic disorders account for a large proportion in the western population, while chronic HBV infection and exposure to carcinogens such as aflatoxins are the main pathogenic factors of liver cancer in the Chinese population.
Although the previous international Pan-Cancer Analysis of Whole Genomes (PCAWG) and The Cancer Genome Atlas (TCGA) included studies on the genome-wide characteristics of liver cancer, their samples were mainly Japanese and western populations, and their results could not fully reflect the genomic characteristics of liver cancer in Chinese population. On the other hand, these studies have adopted relatively shallow sequencing depth (~30-40x), which limits its deep resolution of subclonal structure at the genome-wide level. In view of the huge differences in the pathogenic factors of liver cancer in different regions and different populations, it is urgent to carry out a large-scale, high-depth analysis of the whole gene characteristics of Chinese liver cancer population, so as to provide a basis for the development of diagnosis and treatment programs suitable for Chinese liver cancer population.
Method & Result
Previous genomic analyses of HCC in Chinese individuals are limited to cohort size and focus mainly on exomes, precluding detailed studies at the genome-wide level. Recently, the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium has analyzed the genomic complexity of cancer on a considerable scale. However, the relatively shallow sequencing depth cannot fully resolve the subclonal structure of HCC genome. In CLCA, researchers performed deep whole genome sequencing (WGS) analysis on 494 HCC tumors (average depth, 120×) as well as matched control blood samples (average depth, 36×). The cohort included 427 men (86.4%) and 67 women (13.6%). Compared with the PCAWG-HCC (n=248) cohort, the CLCA cohort had a higher proportion of HBV infection (94.5% vs. 30.6%) and Edmonson-Steiner grades 3 and 4 (85.6% vs. 12.1%), but a lower proportion of hepatitis C virus (HCV) infection (2.6% vs. 55.6%), alcohol consumption (26.7% vs. 58.1%), and smoking (36.8% vs. 53.6%). These statistical data represent the epidemiology of Chinese population with liver cancer, highlighting the necessity of current research.
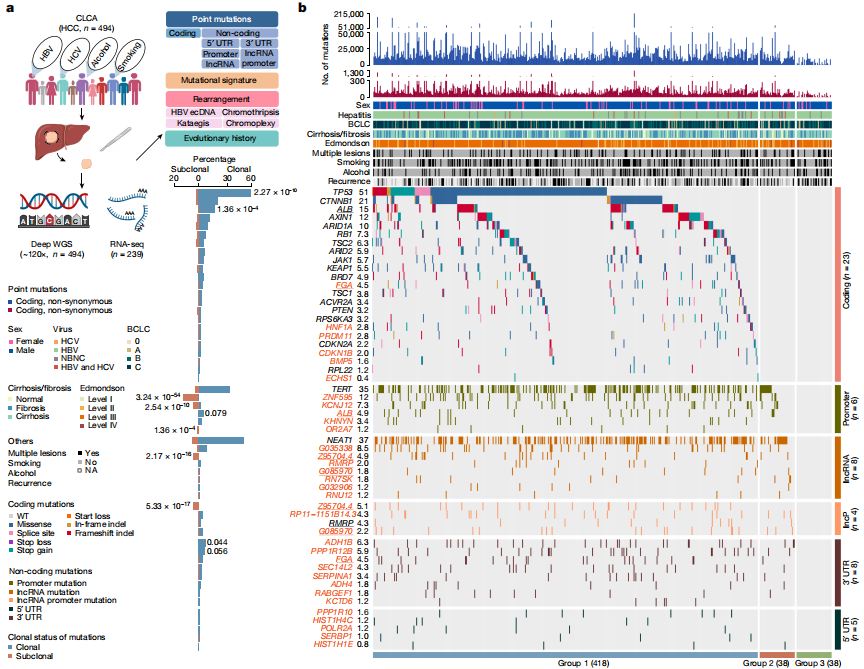
Figure 1. Candidate driver landscape
After strict quality control, a total of 9,287,828 somatic mutations were identified, with a median of 13,735.5 mutations and 95 nonsynonymous mutations per tumor. The researchers also performed RNA sequencing (RNA-seq) analysis on 239 tumors from this cohort. The study identified 6 coding and 28 non-coding previously undescribed driver candidate genes. The study found five previously described mutational features, including aristolochic acid-related indels and doublet-base features, as well as a single-base substitution feature named SBS_H8. Pentanucleotide acid background analysis and experimental validation confirmed that SBS_H8 was different from aristolochic acid-related SBS22.
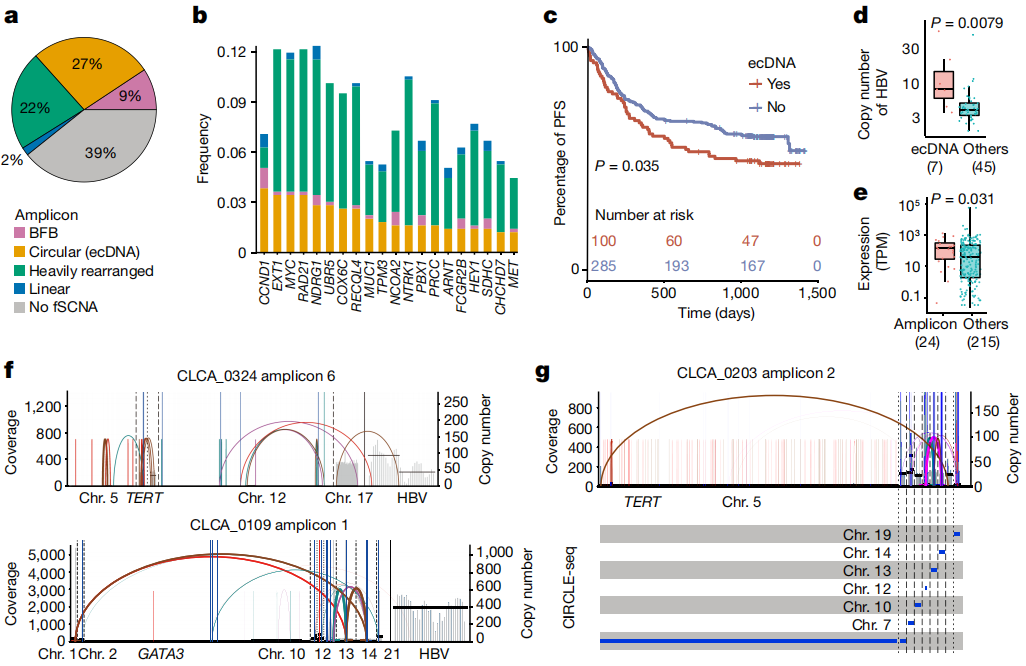
Figure 2. ecDNA analysis
It is worth noting that HBV integration may take the form of extrachromosomal circular DNA, resulting in elevated copy number and gene expression. High depth data also enable researchers to characterize subclonal clustered alterations, including chromothripsis, chromoplexy, and multiple mutation clusters occurring in genomic regions (kataegis), suggesting that these catastrophic events may also occur in the late stage of hepatocarcinogenesis. Pathway analysis of all types of alterations further linked non-coding mutations to hepatic metabolic dysregulation. Finally, the study conducted in vitro and in vivo experiments to demonstrate that FGA, as a candidate coding and noncoding driver, regulates HCC progression and metastasis.
Conclusion
In conclusion, the comprehensive genomic landscape of HCC in Chinese individuals established by this institute provides valuable resources for in-depth understanding of the molecular mechanism of the occurrence and evolution of liver cancer with different etiologies, and the development of new individualized targeted drugs, which helps to improve the level of clinical precision diagnosis and treatment of liver cancer in China and benefit the majority of liver cancer patients.








Nature|Ubigene's KO cells assist in revealing genomic landscape and evolutionary history of HCC in Chinese individuals

Abstract
On February 14, 2024, the research group led by Hongyang Wang from National Center for Liver Cancer/Eastern Hepatobiliary Surgery Hospital, Shanghai, China and the Biomedical Pioneering Innovation Center (BIOPIC), Beijing Advanced Innovation Center for Genomics (ICG), School of Life Sciences, Peking University, Beijing, China jointly published the latest research results entitled Deep whole-genome analysis of 494 hepatocellular carcinomas on Nature, and completed the Chinese Liver Cancer Atlas (CLCA). In this study, 494 HCC patients' tumor tissues from different regions of China were subjected to high-depth whole genome sequencing (average depth, 120x), and the coding and non-coding driver genes, mutational signatures, copy number variations, clustered alternation: chromothripsis, chromoplexy and kataegis, extrachromosomal circular DNA (ecDNA), and the characteristics of mutation evolution were analyzed in depth. Three newly identified potential driver events were also selected for detailed functional verification. Based on CRISPR site directed mutagenesis and knockdown / knockout experiments in multiple cell lines, it was found that mutations in the above genes were sufficient to cause significant changes in gene expression levels (Among them, PPP1R12B, KCNJ12, FGA knockout cells and overexpression lentiviruses carrying mutations were all constructed by Ubigene), and were involved in regulating various malignant phenotypes of hepatocellular carcinoma, These results confirm the validity of the new driving events found based on data analysis.

Background
The mortality of primary liver cancer ranks third among all cancers in the world. Hepatocellular carcinoma (HCC) is its main subtype, accounting for more than 80% of all cases. More than half of the new cases of liver cancer in the world occur in China every year, and the prognosis of patients is generally poor. The five-year overall survival rate of patients with hepatocellular carcinoma in China is only about 10%. The etiology of hepatocellular carcinoma is complex, among which HCV infection is the most common in the Japanese population. Alcohol, non-alcoholic steatohepatitis and metabolic disorders account for a large proportion in the western population, while chronic HBV infection and exposure to carcinogens such as aflatoxins are the main pathogenic factors of liver cancer in the Chinese population.
Although the previous international Pan-Cancer Analysis of Whole Genomes (PCAWG) and The Cancer Genome Atlas (TCGA) included studies on the genome-wide characteristics of liver cancer, their samples were mainly Japanese and western populations, and their results could not fully reflect the genomic characteristics of liver cancer in Chinese population. On the other hand, these studies have adopted relatively shallow sequencing depth (~30-40x), which limits its deep resolution of subclonal structure at the genome-wide level. In view of the huge differences in the pathogenic factors of liver cancer in different regions and different populations, it is urgent to carry out a large-scale, high-depth analysis of the whole gene characteristics of Chinese liver cancer population, so as to provide a basis for the development of diagnosis and treatment programs suitable for Chinese liver cancer population.
Method & Result
Previous genomic analyses of HCC in Chinese individuals are limited to cohort size and focus mainly on exomes, precluding detailed studies at the genome-wide level. Recently, the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium has analyzed the genomic complexity of cancer on a considerable scale. However, the relatively shallow sequencing depth cannot fully resolve the subclonal structure of HCC genome. In CLCA, researchers performed deep whole genome sequencing (WGS) analysis on 494 HCC tumors (average depth, 120×) as well as matched control blood samples (average depth, 36×). The cohort included 427 men (86.4%) and 67 women (13.6%). Compared with the PCAWG-HCC (n=248) cohort, the CLCA cohort had a higher proportion of HBV infection (94.5% vs. 30.6%) and Edmonson-Steiner grades 3 and 4 (85.6% vs. 12.1%), but a lower proportion of hepatitis C virus (HCV) infection (2.6% vs. 55.6%), alcohol consumption (26.7% vs. 58.1%), and smoking (36.8% vs. 53.6%). These statistical data represent the epidemiology of Chinese population with liver cancer, highlighting the necessity of current research.

Figure 1. Candidate driver landscape
After strict quality control, a total of 9,287,828 somatic mutations were identified, with a median of 13,735.5 mutations and 95 nonsynonymous mutations per tumor. The researchers also performed RNA sequencing (RNA-seq) analysis on 239 tumors from this cohort. The study identified 6 coding and 28 non-coding previously undescribed driver candidate genes. The study found five previously described mutational features, including aristolochic acid-related indels and doublet-base features, as well as a single-base substitution feature named SBS_H8. Pentanucleotide acid background analysis and experimental validation confirmed that SBS_H8 was different from aristolochic acid-related SBS22.

Figure 2. ecDNA analysis
It is worth noting that HBV integration may take the form of extrachromosomal circular DNA, resulting in elevated copy number and gene expression. High depth data also enable researchers to characterize subclonal clustered alterations, including chromothripsis, chromoplexy, and multiple mutation clusters occurring in genomic regions (kataegis), suggesting that these catastrophic events may also occur in the late stage of hepatocarcinogenesis. Pathway analysis of all types of alterations further linked non-coding mutations to hepatic metabolic dysregulation. Finally, the study conducted in vitro and in vivo experiments to demonstrate that FGA, as a candidate coding and noncoding driver, regulates HCC progression and metastasis.
Conclusion
In conclusion, the comprehensive genomic landscape of HCC in Chinese individuals established by this institute provides valuable resources for in-depth understanding of the molecular mechanism of the occurrence and evolution of liver cancer with different etiologies, and the development of new individualized targeted drugs, which helps to improve the level of clinical precision diagnosis and treatment of liver cancer in China and benefit the majority of liver cancer patients.








