Location:Home > Application > IF=16.6| 4T1 Luciferase in Breast Cancer Metastasis Study
IF=16.6| Ubigene's Luciferase Cell Aids in the Study of Disseminated Breast Cancer Metastasis
Almost all breast cancer-related deaths can be attributed to metastasis, and even before metastasis occurs, the suitable “soil” for tumor cell seeds to settle, known as the pre-metastatic niche (PMN), is already established in distant organs. PMN continuously releases chemokines into the circulatory system, allowing tumor cells to selectively migrate to the distant organs where PMN is present. The chemokine receptor CXCR4 usually exists on the surface of tumor cells as monomers, dimers, or polymers, and stimulation by the chemokine CXCL12 can alter the dynamic equilibrium of different CXCR4 conformations, enabling cells to correctly perceive the chemokine gradient and regulate the direction of migration. Currently, only two CXCR4 antagonists, the bicyclam small molecule AMD3100 and the cyclic peptide BKT140, are on the market, both of which rely on the interaction with the monomeric receptor. Based on this background, researchers wondered whether CXCR4 spatial distribution could be altered by mechanical traction to disrupt the dynamic equilibrium between CXCR4 monomers, dimers, and polymers to block tumor metastasis.
Recently, the research group led by Professor Lian Li from West China School of Pharmacy, Sichuan University, China, published an original research paper titled “Cell surface patching via CXCR4-targeted nanothreads for cancer metastasis inhibition” on Nature Communications(IF: 16.6). The authors developed a network-like cross-linking strategy that induces wide-scale CXCR4 clusteringon the cell surface, effectively inhibiting spontaneous metastatic tumors and disseminated metastatic tumors. [1]. This study used the luciferase expressing mouse breast cancer cell line (4T1-Luc) provided by Ubigene as the pharmacological evaluation model for inhibiting disseminated tumor metastasis.
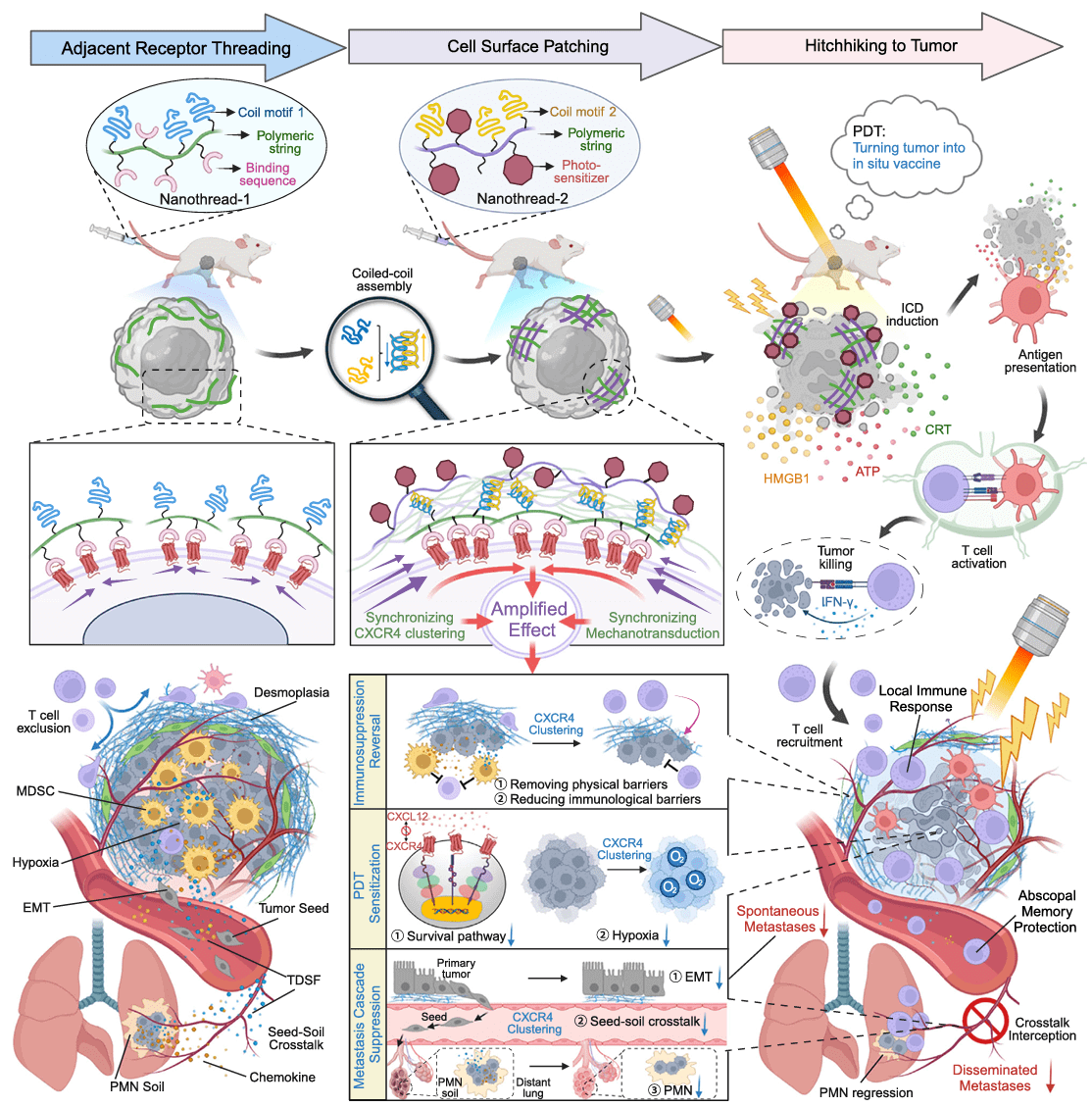
Figure 1. Schematic diagram of network cross-linking CXCR4 receptor strategy combined with photodynamic therapy to inhibit spontaneous metastatic tumors and disseminated metastatic tumors
Nanothread-1 labeled with red fluorescence anchors uniformly on the cell surface after the first administration. Subsequently, Nanothread-2 labeled with green fluorescence was administered, and it was observed that the red fluorescence and green fluorescence highly overlapped, and their distribution on the cell surface underwent significant changes from a uniform circular pattern to a concentrated dot pattern. This indicates that the two polymers interact on the cell surface, leading to receptor redistribution. To further demonstrate receptor clustering, cells were transfected to express fluorescently labeled CXCR4. Compared to the untreated group, free ligand, and polymer-based multivalent ligand cross-linking strategy, the receptor had a stronger effect, significantly inducing receptor clustering and altering the spatial distribution of CXCR4 on the cell surface.
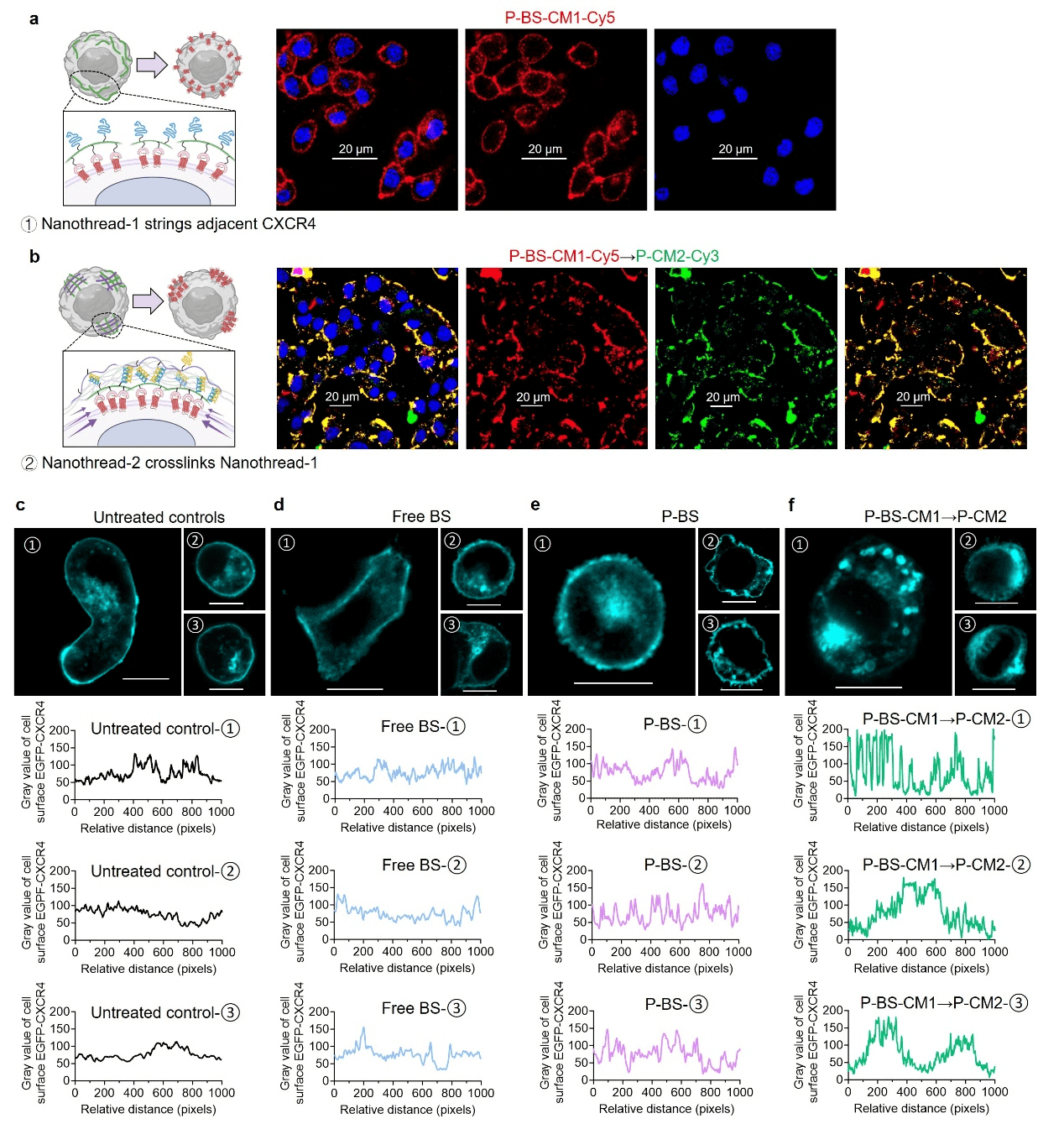
Figure 2. Network cross-linking strategy inducing CXCR4 clustering on the cell surface
Furthermore, the authors found that different CXCR4 antagonistic modes had different intervention capabilities on downstream pro-metastatic pathways, with the receptor cross-linking antagonistic mode significantly outperforming multivalent ligands or free ligands. Cross-linking receptor clustering could effectively mediate tumor seed phenotype reshaping, seed-soil communication interception, PMN soil degradation, block key processes in the metastasis cascade, and confine cancer cells to the original tumor site. In addition, the authors covalently linked a photosensitizer to Nanothread-2 to achieve tumor-targeted photodynamic therapy in a “hitchhiking” manner, not only enhancing killing of the primary tumor but also significantly inducing immunogenic cell death and activating anti-tumor immune responses. Mice injected intravenously with the bioluminescent mouse breast cancer cells (4T1-Luc) provided by Ubigene were used to establish a model of disseminated tumor metastasis. Post-combined therapy, the mice resisted the growth and spread of disseminated tumor cells in vivo, and the effector memory T cells increased significantly. This indicates that the above strategy can simultaneously inhibit spontaneous metastatic tumors and disseminated metastatic tumors.
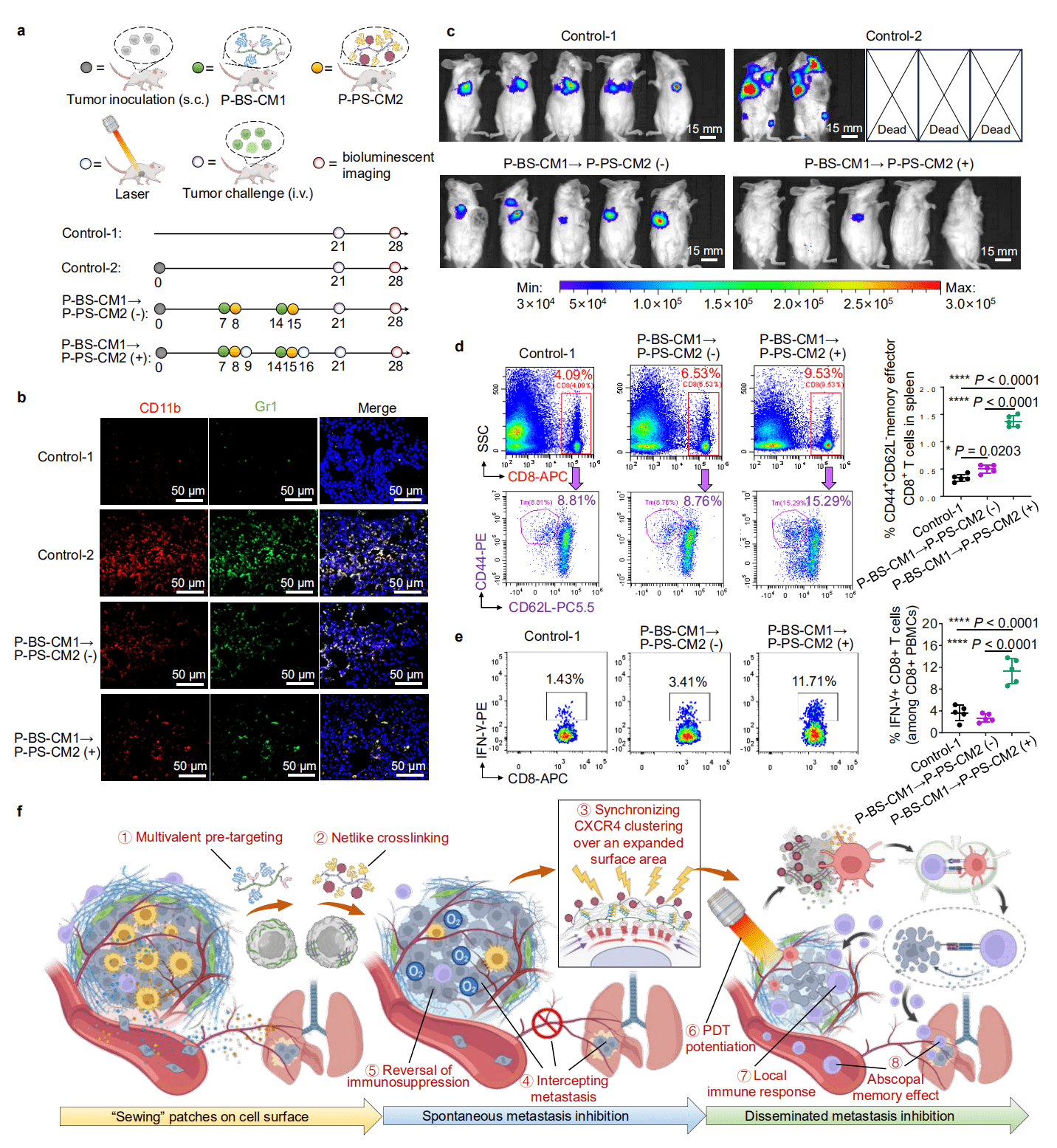
Luciferase expressing cell lines are used in vivo imaging in animal models, allowing real-time monitoring of disease progression and treatment effects, demonstrating great potential and value in various research studies. Ubigene’s Luciferase cell lines stably express firefly luciferase, are highly sensitive and specific, and has high activity with good condition. They can be directly used for in vivo cell injection experiments, helping with studies on transcription factor regulation mechanisms, in vivo imaging, cell tracking, and more. Ubigene not only has various Luciferase cell lines in stock but also offers custom Luciferase cell line services. Feel free to inquire!
Referrences
[1] Zhou, M., Liu, C., Li, B., Li, J., Zhang, P., Huang, Y., Li, L. Cell surface patching via CXCR4-targeted nanothreads for cancer metastasis inhibition. Nat Commun. 2024 15:2763. doi: 10.1038/s41467-024-47111-z


IF=16.6| Ubigene's Luciferase Cell Aids in the Study of Disseminated Breast Cancer Metastasis

Almost all breast cancer-related deaths can be attributed to metastasis, and even before metastasis occurs, the suitable “soil” for tumor cell seeds to settle, known as the pre-metastatic niche (PMN), is already established in distant organs. PMN continuously releases chemokines into the circulatory system, allowing tumor cells to selectively migrate to the distant organs where PMN is present. The chemokine receptor CXCR4 usually exists on the surface of tumor cells as monomers, dimers, or polymers, and stimulation by the chemokine CXCL12 can alter the dynamic equilibrium of different CXCR4 conformations, enabling cells to correctly perceive the chemokine gradient and regulate the direction of migration. Currently, only two CXCR4 antagonists, the bicyclam small molecule AMD3100 and the cyclic peptide BKT140, are on the market, both of which rely on the interaction with the monomeric receptor. Based on this background, researchers wondered whether CXCR4 spatial distribution could be altered by mechanical traction to disrupt the dynamic equilibrium between CXCR4 monomers, dimers, and polymers to block tumor metastasis.
Recently, the research group led by Professor Lian Li from West China School of Pharmacy, Sichuan University, China, published an original research paper titled “Cell surface patching via CXCR4-targeted nanothreads for cancer metastasis inhibition” on Nature Communications(IF: 16.6). The authors developed a network-like cross-linking strategy that induces wide-scale CXCR4 clusteringon the cell surface, effectively inhibiting spontaneous metastatic tumors and disseminated metastatic tumors. [1]. This study used the luciferase expressing mouse breast cancer cell line (4T1-Luc) provided by Ubigene as the pharmacological evaluation model for inhibiting disseminated tumor metastasis.

Figure 1. Schematic diagram of network cross-linking CXCR4 receptor strategy combined with photodynamic therapy to inhibit spontaneous metastatic tumors and disseminated metastatic tumors
Nanothread-1 labeled with red fluorescence anchors uniformly on the cell surface after the first administration. Subsequently, Nanothread-2 labeled with green fluorescence was administered, and it was observed that the red fluorescence and green fluorescence highly overlapped, and their distribution on the cell surface underwent significant changes from a uniform circular pattern to a concentrated dot pattern. This indicates that the two polymers interact on the cell surface, leading to receptor redistribution. To further demonstrate receptor clustering, cells were transfected to express fluorescently labeled CXCR4. Compared to the untreated group, free ligand, and polymer-based multivalent ligand cross-linking strategy, the receptor had a stronger effect, significantly inducing receptor clustering and altering the spatial distribution of CXCR4 on the cell surface.

Figure 2. Network cross-linking strategy inducing CXCR4 clustering on the cell surface
Furthermore, the authors found that different CXCR4 antagonistic modes had different intervention capabilities on downstream pro-metastatic pathways, with the receptor cross-linking antagonistic mode significantly outperforming multivalent ligands or free ligands. Cross-linking receptor clustering could effectively mediate tumor seed phenotype reshaping, seed-soil communication interception, PMN soil degradation, block key processes in the metastasis cascade, and confine cancer cells to the original tumor site. In addition, the authors covalently linked a photosensitizer to Nanothread-2 to achieve tumor-targeted photodynamic therapy in a “hitchhiking” manner, not only enhancing killing of the primary tumor but also significantly inducing immunogenic cell death and activating anti-tumor immune responses. Mice injected intravenously with the bioluminescent mouse breast cancer cells (4T1-Luc) provided by Ubigene were used to establish a model of disseminated tumor metastasis. Post-combined therapy, the mice resisted the growth and spread of disseminated tumor cells in vivo, and the effector memory T cells increased significantly. This indicates that the above strategy can simultaneously inhibit spontaneous metastatic tumors and disseminated metastatic tumors.

Luciferase expressing cell lines are used in vivo imaging in animal models, allowing real-time monitoring of disease progression and treatment effects, demonstrating great potential and value in various research studies. Ubigene’s Luciferase cell lines stably express firefly luciferase, are highly sensitive and specific, and has high activity with good condition. They can be directly used for in vivo cell injection experiments, helping with studies on transcription factor regulation mechanisms, in vivo imaging, cell tracking, and more. Ubigene not only has various Luciferase cell lines in stock but also offers custom Luciferase cell line services. Feel free to inquire!
Referrences
[1] Zhou, M., Liu, C., Li, B., Li, J., Zhang, P., Huang, Y., Li, L. Cell surface patching via CXCR4-targeted nanothreads for cancer metastasis inhibition. Nat Commun. 2024 15:2763. doi: 10.1038/s41467-024-47111-z

