Location:Home > Application > The Powerful Function Of The Gene-Editing NK-92 Cell Line
The Powerful Function Of The Gene-Editing NK-92 Cell Line

Natural killer (NK) cells are composed of a unique group of innate lymphoid cells with various cytotoxic mechanisms and the ability to regulate immune responses through cytokines, playing a key role in cancer immunotherapy[1]. Their importance and potential have been widely studied over the past fifty years[2]. Initially a subject of academic exploration, it has now evolved into a promising field of cancer immunotherapy.
NK-92 cells are IL-2-dependent NK cell lines from peripheral blood mononuclear cells isolated from acute non-Hodgkin's lymphoma. Among many NK cells, NK-92 cells are considered candidates for “ready-to-use” NK cell adoptive immunotherapy due to their ease of culture and expansion. NK-92 cells are also the first NK cells to be approved by the U.S. Food and Drug Administration (FDA) for clinical trials[1]. However, researchers have found that NK-92 cells often experience functional exhaustion in the tumor microenvironment. To overcome immuno-suppression or enhance NK cell tumor targeting recognition, various methods have been developed. Among them, researchers can use gene-editing to engineer NK-92 cells to enhance NK cell adaptability and anti-tumor capabilities[3]. Follow us to see how researchers modified the NK-92 cells!
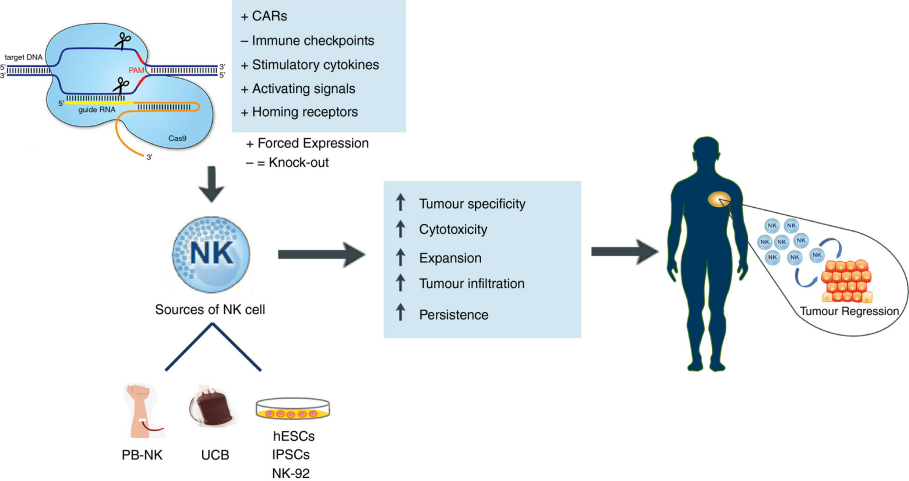
Figure 1 Schematic overview of the CRISPR/Cas9‐mediated genetic reprogramming of NK cells for cancer immunotherapy[3]
“CAR-loaded” NK Cell
Chimeric antigen receptor T cells (CAR-T cells) therapy has shown
significant efficacy in treating leukemia, but personalized customization and high costs remain a challenge.
Therefore, developing a more affordable and readily available treatment is crucial. Guillermo et al.[4] explored the potential of CRISPR-Cas9
technology-modified CAR-NK-92 cells in treating acute myeloid leukemia (AML) and acute B-cell lymphoblastic
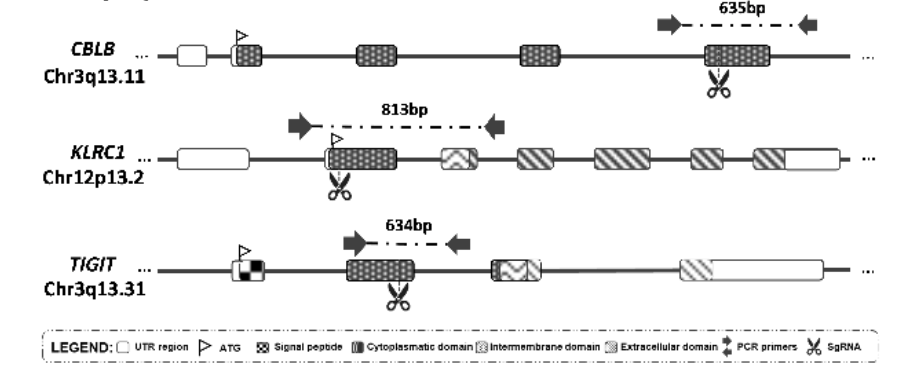
leukemia (B-ALL). Firstly, NK-92 cells were modified to express specific chimeric antigen receptors (CAR) that can recognize and target cancer cells. Subsequently, immune checkpoint genes (CBLB, NKG2A, TIGIT) on
NK cells were knocked out, aiming to enhance the cytotoxicity of NK-92 cells. The
experimental results showed that compared to unmodified NK-92 cells, CRISPR-edited CAR-NK-92 cells exhibited
stronger targeted cytotoxicity in in vitro experiments, especially in AML cell lines. Although the cytotoxic
effects were not significant in B-ALL cell lines, the triple knockout
CD276-CAR-NK-92 cells demonstrated significantly enhanced cytotoxicity in AML treatment,
indicating the potential of these engineered NK-92 cells to be an effective treatment. This study highlights the
application potential of NK92 cells in cancer immunotherapy, particularly demonstrating enhanced cytotoxicity
against leukemia cells after editing with CRISPR-Cas9 technology.
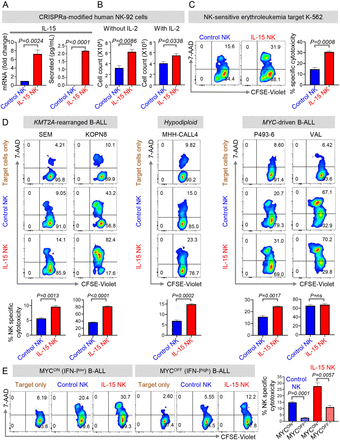
Enhancing NK Cell Activation Pathways
Type I interferon (IFN-I) plays a driving role in immune
surveillance of solid tumors. However, the IFN-I-driven immune response is inhibited in hematologic
malignancies. To investigate the impact of this mechanism on disease development, Kumar Anil et
al.[5] conducted a study and explored the
therapeutic potential of IL-15-secreting NK cells. They found that high expression of the IFN-I
signaling pathway was associated with favorable clinical outcomes in B-ALL patients, but IFN-I production was intrinsically suppressed in the B-ALL
microenvironment, leading to reduced IL-15 and diminished NK cell function. Using CRISPRa technology, researchers created NK-92 cells capable of secreting IL-15. The
results showed that when targeting MYC-overexpressing B-ALL cells, these IL-15-secreting
NK-92 cells exhibited increased proliferation capacity and cytotoxicity. In in vivo models, IL-15-secreting NK-92 cells effectively slowed the progression of
MYC-overexpressing B-ALL, indicating their potential therapeutic application in restoring
IFN-I pathway activity and enhancing NK cell-mediated immune surveillance.
Discovery of CISH Target for Treatment
Current immunotherapy strategies mainly target CD8+ T cells, but many
patients are insensitive to these treatments. Therefore, researchers have begun to explore the potential
therapeutic roles of other immune cells. In T cells, inhibition of the CISH gene has been shown to enhance
anti-tumor immune responses. Therefore, researchers believe that by knocking out the CISH gene, they can improve
the natural cytotoxicity receptor (NCR) signaling function of NK cells, thereby enhancing NK cells' recognition
and killing abilities against tumor cells. Bernard Pierre-Louis et al.[6] created a conditional CISH-deficient mouse
model (Cishfl/flNcr1Ki/+) to assess the role of CISH in NK cell function and anti-tumor
properties. They used CRISPRi technology to knock out the CISH gene in the NK-92 cell
line to explore the impact of CISH on NK cell function. The results showed that knocking out CISH enhanced NCR signaling in NK-92 cells, increased their anti-tumor function, and
reduced the expression of TIGIT, which may help reduce NK cell exhaustion. These findings suggest
that CISH is an emerging therapeutic target that can be used to enhance the efficacy of NK cell immunotherapy.
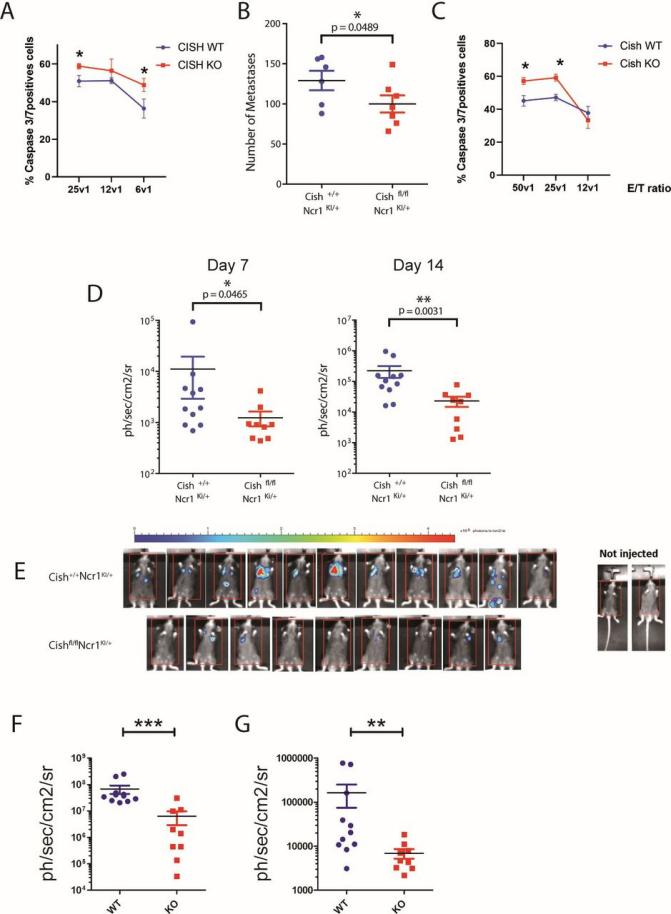
Figure 4 NK cells with CISH deficiency have an enhanced immune response against "metastasis"[6]
From the above studies, it is evident that gene-edited NK cells have
powerful potential in combating tumors. However, NK cells are resistant to conventional
transfection methods, making the delivery of Cas9 systems challenging, which is a key issue in current
research on gene-edited NK cells. Now, Ubigene has
successfully constructed a NK-92 cell line suitable for gene editing,
which have been validated through gene-editing cases! If you encounter difficulties with NK-92 cells' transfection
and other gene-editing issues during your research, hand over your project to Ubigene, let’s help you solve the isssues easily!
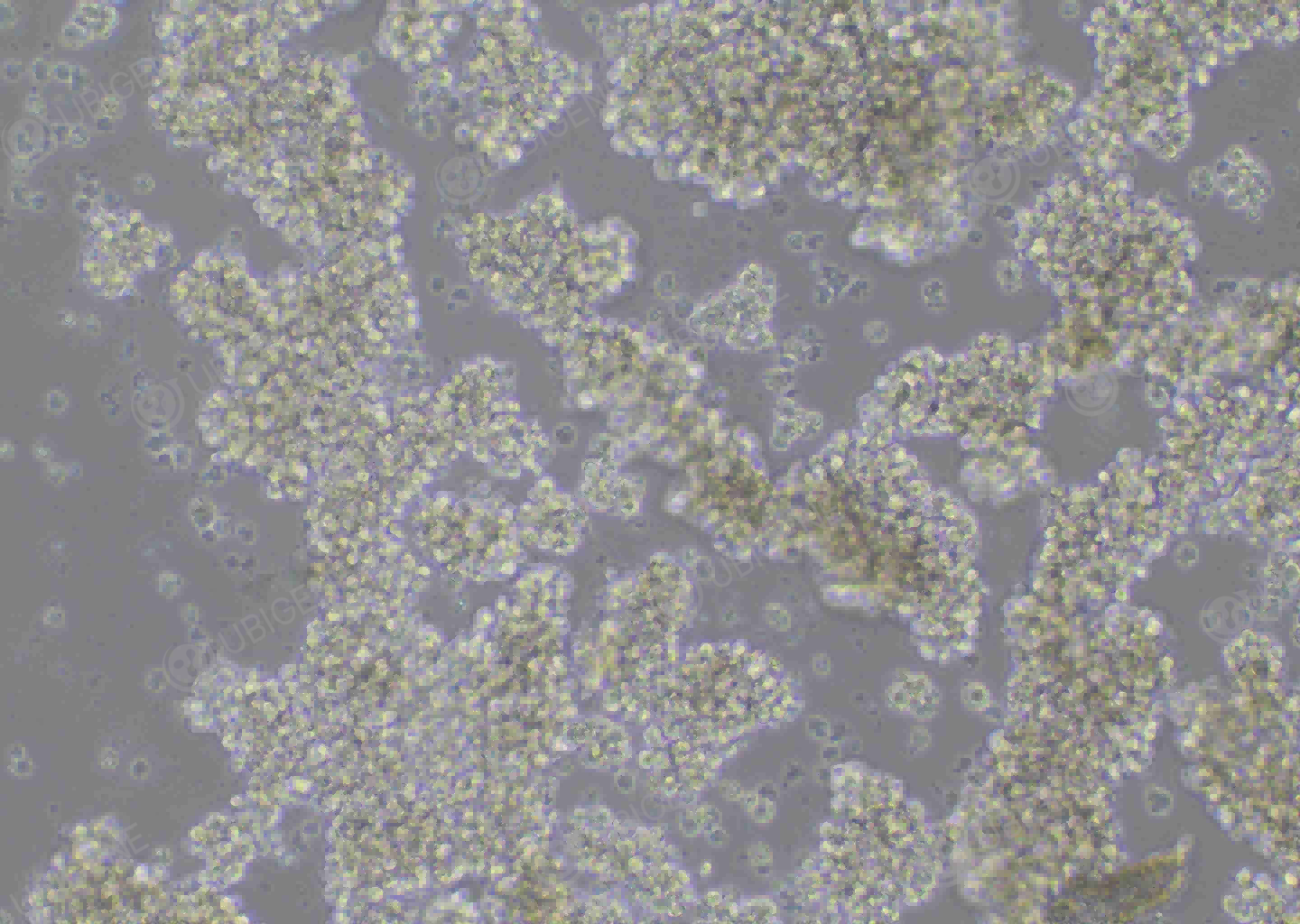
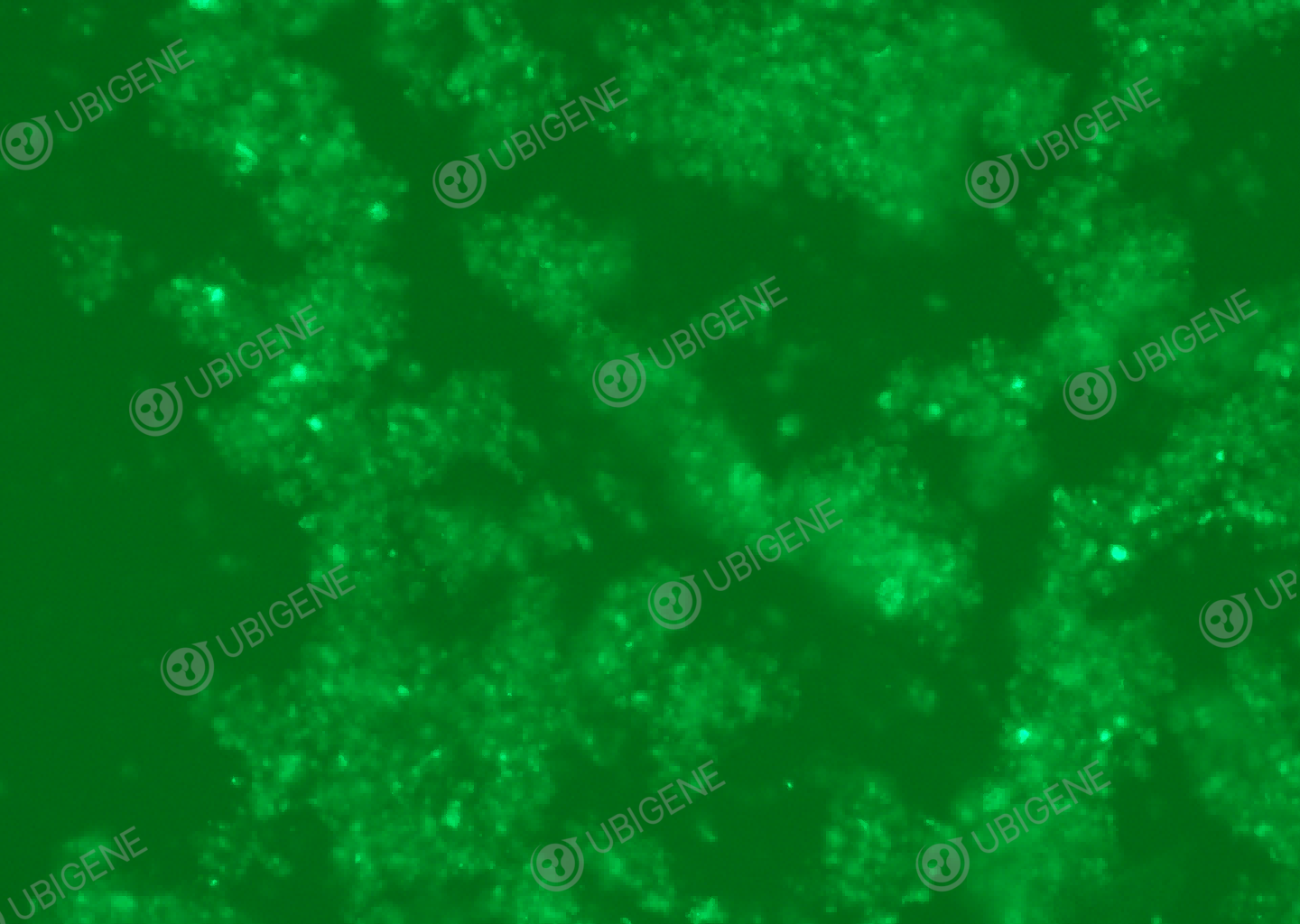
Figure 5 Cell image of the NK-92 cells with gene knockout by Ubigene
References
[1] Laskowski,
Tamara J., Alexander Biederstädt, and Katayoun Rezvani. "Natural killer cells in antitumour adoptive cell
immunotherapy." Nature Reviews Cancer 22.10 (2022): 557-575.
Nature:Natural killer cell therapies
[2] Vivier, Eric, et al. "Natural killer cell therapies." Nature 626.8000
(2024): 727-736.
[3] Afolabi,
Lukman O., et al. "Genetic reprogramming for NK cell cancer immunotherapy with CRISPR/Cas9." Immunology 158.2 (2019): 63-69.
[4] Ureña-Bailén, Guillermo, et al. "Preclinical evaluation of CRISPR-edited
CAR-NK-92 cells for off-the-shelf treatment of AML and B-ALL." International Journal of
Molecular Sciences 23.21 (2022): 12828.
[5] Kumar, Anil, et al. "Intrinsic suppression of type I interferon production
underlies the therapeutic efficacy of il-15-producing natural killer cells in B-cell acute lymphoblastic
leukemia." Journal for Immunotherapy of Cancer 11.5 (2023).
Bernard, Pierre-Louis, et al. "Targeting CISH enhances natural cytotoxicity receptor signaling and reduces NK cell exhaustion to improve solid tumor immunity." Journal for Immunotherapy of Cancer 10.5 (2022).


The Powerful Function Of The Gene-Editing NK-92 Cell Line

Natural killer (NK) cells are composed of a unique group of innate lymphoid cells with various cytotoxic mechanisms and the ability to regulate immune responses through cytokines, playing a key role in cancer immunotherapy[1]. Their importance and potential have been widely studied over the past fifty years[2]. Initially a subject of academic exploration, it has now evolved into a promising field of cancer immunotherapy.
NK-92 cells are IL-2-dependent NK cell lines from peripheral blood mononuclear cells isolated from acute non-Hodgkin's lymphoma. Among many NK cells, NK-92 cells are considered candidates for “ready-to-use” NK cell adoptive immunotherapy due to their ease of culture and expansion. NK-92 cells are also the first NK cells to be approved by the U.S. Food and Drug Administration (FDA) for clinical trials[1]. However, researchers have found that NK-92 cells often experience functional exhaustion in the tumor microenvironment. To overcome immuno-suppression or enhance NK cell tumor targeting recognition, various methods have been developed. Among them, researchers can use gene-editing to engineer NK-92 cells to enhance NK cell adaptability and anti-tumor capabilities[3]. Follow us to see how researchers modified the NK-92 cells!

Figure 1 Schematic overview of the CRISPR/Cas9‐mediated genetic reprogramming of NK cells for cancer immunotherapy[3]
“CAR-loaded” NK Cell
Chimeric antigen receptor T cells (CAR-T cells) therapy has shown
significant efficacy in treating leukemia, but personalized customization and high costs remain a challenge.
Therefore, developing a more affordable and readily available treatment is crucial. Guillermo et al.[4] explored the potential of CRISPR-Cas9
technology-modified CAR-NK-92 cells in treating acute myeloid leukemia (AML) and acute B-cell lymphoblastic
leukemia (B-ALL). Firstly, NK-92 cells were modified to express specific chimeric antigen receptors (CAR) that can recognize and target cancer cells. Subsequently, immune checkpoint genes (CBLB, NKG2A, TIGIT) on
NK cells were knocked out, aiming to enhance the cytotoxicity of NK-92 cells. The
experimental results showed that compared to unmodified NK-92 cells, CRISPR-edited CAR-NK-92 cells exhibited
stronger targeted cytotoxicity in in vitro experiments, especially in AML cell lines. Although the cytotoxic
effects were not significant in B-ALL cell lines, the triple knockout
CD276-CAR-NK-92 cells demonstrated significantly enhanced cytotoxicity in AML treatment,
indicating the potential of these engineered NK-92 cells to be an effective treatment. This study highlights the
application potential of NK92 cells in cancer immunotherapy, particularly demonstrating enhanced cytotoxicity
against leukemia cells after editing with CRISPR-Cas9 technology.

Enhancing NK Cell Activation Pathways
Type I interferon (IFN-I) plays a driving role in immune
surveillance of solid tumors. However, the IFN-I-driven immune response is inhibited in hematologic
malignancies. To investigate the impact of this mechanism on disease development, Kumar Anil et
al.[5] conducted a study and explored the
therapeutic potential of IL-15-secreting NK cells. They found that high expression of the IFN-I
signaling pathway was associated with favorable clinical outcomes in B-ALL patients, but IFN-I production was intrinsically suppressed in the B-ALL
microenvironment, leading to reduced IL-15 and diminished NK cell function. Using CRISPRa technology, researchers created NK-92 cells capable of secreting IL-15. The
results showed that when targeting MYC-overexpressing B-ALL cells, these IL-15-secreting
NK-92 cells exhibited increased proliferation capacity and cytotoxicity. In in vivo models, IL-15-secreting NK-92 cells effectively slowed the progression of
MYC-overexpressing B-ALL, indicating their potential therapeutic application in restoring
IFN-I pathway activity and enhancing NK cell-mediated immune surveillance.

Discovery of CISH Target for Treatment
Current immunotherapy strategies mainly target CD8+ T cells, but many
patients are insensitive to these treatments. Therefore, researchers have begun to explore the potential
therapeutic roles of other immune cells. In T cells, inhibition of the CISH gene has been shown to enhance
anti-tumor immune responses. Therefore, researchers believe that by knocking out the CISH gene, they can improve
the natural cytotoxicity receptor (NCR) signaling function of NK cells, thereby enhancing NK cells' recognition
and killing abilities against tumor cells. Bernard Pierre-Louis et al.[6] created a conditional CISH-deficient mouse
model (Cishfl/flNcr1Ki/+) to assess the role of CISH in NK cell function and anti-tumor
properties. They used CRISPRi technology to knock out the CISH gene in the NK-92 cell
line to explore the impact of CISH on NK cell function. The results showed that knocking out CISH enhanced NCR signaling in NK-92 cells, increased their anti-tumor function, and
reduced the expression of TIGIT, which may help reduce NK cell exhaustion. These findings suggest
that CISH is an emerging therapeutic target that can be used to enhance the efficacy of NK cell immunotherapy.

Figure 4 NK cells with CISH deficiency have an enhanced immune response against "metastasis"[6]
From the above studies, it is evident that gene-edited NK cells have
powerful potential in combating tumors. However, NK cells are resistant to conventional
transfection methods, making the delivery of Cas9 systems challenging, which is a key issue in current
research on gene-edited NK cells. Now, Ubigene has
successfully constructed a NK-92 cell line suitable for gene editing,
which have been validated through gene-editing cases! If you encounter difficulties with NK-92 cells' transfection
and other gene-editing issues during your research, hand over your project to Ubigene, let’s help you solve the isssues easily!


Figure 5 Cell image of the NK-92 cells with gene knockout by Ubigene
References
[1] Laskowski,
Tamara J., Alexander Biederstädt, and Katayoun Rezvani. "Natural killer cells in antitumour adoptive cell
immunotherapy." Nature Reviews Cancer 22.10 (2022): 557-575.
Nature:Natural killer cell therapies
[2] Vivier, Eric, et al. "Natural killer cell therapies." Nature 626.8000
(2024): 727-736.
[3] Afolabi,
Lukman O., et al. "Genetic reprogramming for NK cell cancer immunotherapy with CRISPR/Cas9." Immunology 158.2 (2019): 63-69.
[4] Ureña-Bailén, Guillermo, et al. "Preclinical evaluation of CRISPR-edited
CAR-NK-92 cells for off-the-shelf treatment of AML and B-ALL." International Journal of
Molecular Sciences 23.21 (2022): 12828.
[5] Kumar, Anil, et al. "Intrinsic suppression of type I interferon production
underlies the therapeutic efficacy of il-15-producing natural killer cells in B-cell acute lymphoblastic
leukemia." Journal for Immunotherapy of Cancer 11.5 (2023).
Bernard, Pierre-Louis, et al. "Targeting CISH enhances natural cytotoxicity receptor signaling and reduces NK cell exhaustion to improve solid tumor immunity." Journal for Immunotherapy of Cancer 10.5 (2022).


