B16-F10 Knockout (KO) cell line

B16-F10 Cell Line With "Gene Scissors" CRISPR/Cas9
-- A Weapon to Defeat the "Cancer of the King"

B16 F10 Melanoma Cell Line Characteristics
Murine melanoma cell line B16-F10 is derived from spontaneous tumor cells of C57BL/6J mice. It is a mixture of spindle-shaped and epithelial-like cells under the microscope. It can form a clone after a few days by inoculating a few cells. This shows that the B16-F10 cell line has a strong proliferation ability. In addition, it also shows the strong invasiveness and a high tumor formation rate. So it is considered to be an ideal cell line for building tumor models. A lot of researchers will consider using the B16-F10 cell line when building tumor models. In recent years, the incidence rate of melanoma has been increasing. Malignant melanoma (MM) is a malignant melanoma origin from the skin, which shows the rapid metastasis, poor prognosis, and short survival time. According to statistics, the average survival time of patients with advanced melanoma is shorter than 1 year, and the 5-year survival rate is lower than 10%. Therefore, murine melanoma cell line B16-F10 is of great significance for the study of melanoma metastasis and relevant treatment[1].
Ubigene provides WT B16-F10 cell line, which is suitable for various gene-editing experiments. We also provide the stable B16-F10-Luc cell line for in vivo imaging, etc. You can inquire with our technical specialists for more help!
Detailed Applications of B16-F10 Cells
1. In vivo tracking and metastasis
B16-F10 cell line originated from mouse melanin producing epithelial cells, which can be easily tracked in vivo after transplantation. Therefore, B16-F10 cell line has become an effective tool to study the metastasis pathway. At present, many experiments use B16-F10 cell line to study the immune response of cells to vaccines and the characteristics of miRNA mediated metastasis, especially miR-21 (invader of tumor suppressor and anti-proliferative factor).
2. B16-F10 tumor models
In recent years, cancer study researchers have made significant progress by developing appropriate and accurate animal disease models, the most important of these is transplantable rodent tumors. Among the tumor models in cancer research, B16-F10 has become an important research model.
Applications of CRISPR/Cas9 Technology in B16-F10 Cell Line
CRISPR/Cas9 has become a powerful tool to modify the gene and activate or inhibit gene expression. Therefore, CRISPR/Cas9 technology can be used to analyze the mechanism of tumorigenesis and find new targets for drug development. Melanoma is the most invasive skin cancer. Although oncogene targeted drugs and immune checkpoint inhibitors have achieved great success in improving the overall survival rate of patients, the related toxicity and emerging drug resistance are still huge challenges. Therefore, gene therapy has become an attractive option, which can improve the efficacy of the currently available melanoma therapy and improve the prognosis of patients. The studies have found that PTGS2 is expressed in malignant melanoma, and its expression is significantly related to the poor survival rate of patients. So Ercolano et al. used CRISPR/Cas9 technology to study the role of Prostaglandin Endoperoxide Synthase2(PTGS2) in the expression and metastasis of melanoma. Using CRISPR/Cas9 technology, they knocked out PTGS2 from B16-F10 cell line and found that the reduced expression of PTGS2 in melanoma cells not only inhibited the proliferation, migration and invasion of B16-F10 cell line, but also regulated the immune response by weakening myelogenous inhibition of cell differentiation, and finally effectively inhibited the expression and metastasis of tumors in vivo. The results show that PTGS2 can be used as a targeted therapeutic gene for melanoma, which is of great significance for the development of new selective melanoma therapeutic drugs[2]
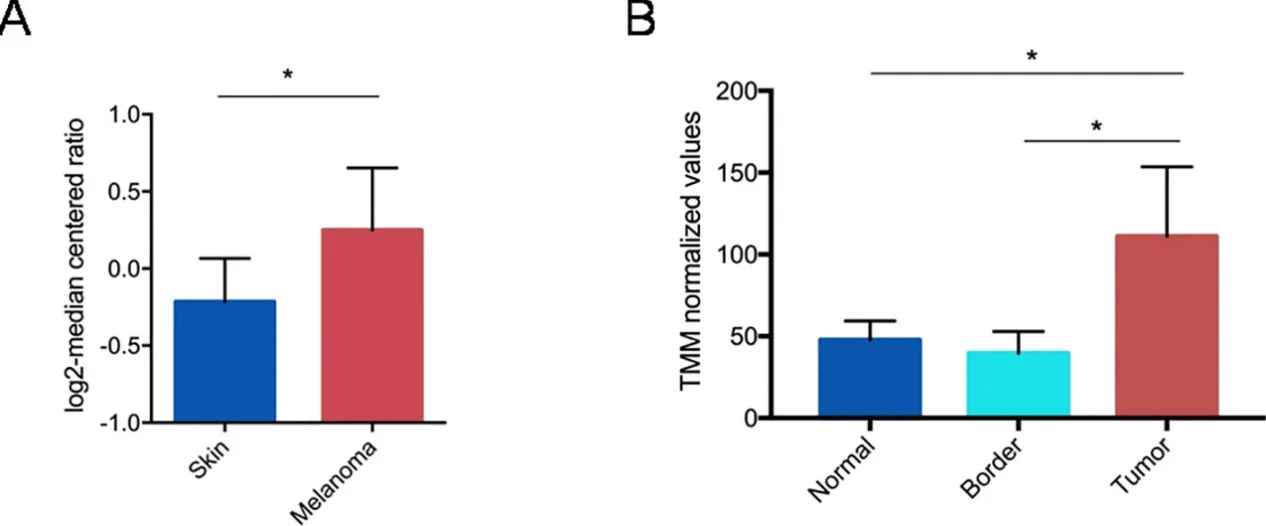
Figure 1 Gene expression analysis of PTGS2
Specific Case Studies of Gene-Editing in B16-F10 Cell Line
1. B16-F10 cell line after knocking out β2m laid the foundation for transplantable tumors.
T lymphocytes of adaptive immune system (T-Cell) recognize short peptides from endogenous cellular proteins or exogenous antigens presented by MHC I and MHC II molecules on the cell surface. Since the formation of stable MHC I/ peptide complexes depends on β2m (light chain), knocking out the β2m in B16-F10 cell line by CRISPR/cas9 system to achieve β2m defective expression and then lead to insufficient expression of MHC molecules on its surface. Das et al. used the CRISPR/Cas9 system to knock out β2m in B16-F10 cell line. And MHC II negative B16-F10 cell line is generated by targeting IAbβ chain coding sites. The results show that MHC I or MHC II deficient tumor cell lines generated by CRISPR/Cas9 technology can be used as parental line to establish MHC compatible transplantable tumor models in HLA transgenic mouse strains lacking endogenous MHC molecular expression[3].
Learn more about custom gene knockout services→
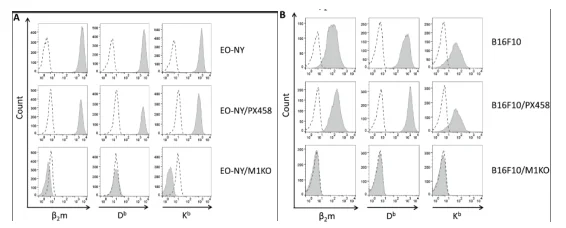
Figure 2 Stable β2m-KO clones phenotype of different tumor entities
2. Jak1 knockout (KO) B16-F10 cell line plays an important role in cancer immunotherapy
Cancer immunotherapy, including immune checkpoint antibody and chimeric antigen receptor T-cell therapy. Although this method has been successful in clinic, a few patients still show drug resistance. Researchers believe that there may be a potential mechanism that can effectively overcome this resistance, and it is crucial for developing more effective cancer treatments. Han et al. established a genome-wide knockout B16-F10 cell line by CRISPR/Cas9 technology. Through in vivo OT-I T-cell transfer and in vitro OT-I T-cell cytotoxic test, it was shown that Janus kinase Jak1 deficiency can mediate T-cell drug resistance. The deletion of Jak1 reduces JAK signal transducer and transcriptional signal activator in B16-F10 cell line, leading to in tumor resistance to T-cell effector molecule interferon and inhibiting T-cell activation by impairing antigen presentation. These findings provide a new method for exploring the drug resistance of tumor immunotherapy, and prove that Jak1 is a potential therapeutic target for the effective treatment of melanoma[4].
3. B16-F10 cell line after point mutation can accurately simulate human pathology
Most human genetic diseases originate from point mutations of G: C>A: T or T: A>C: G base changes, which represent nearly half of the pathogenic single nucleotide polymorphisms (SNPs). The animal model of human genetic diseases is of great significance in analyzing the pathogenesis, drug screening and efficacy test. Gene-editing experiments such as gene knockout and point mutation on B16-F10 cell line using CRISPR/Cas9 system can build a human disease model that can accurately simulate human pathology[5].
Learn more about custom point mutation services→
So far, Ubigene has successfully modified genes from the B16-F10 cell line with our developed CRISPR-UTM technology, and we have successfully built stable B16-F10-Luc cell line, which can be used in vivo imaging and other experiments. If you want to know more information of our products, please feel free to contact us!
Reference
[1] Gerber SA, Sorensen EW, Sedlacek AL, et al. Local expression of interleukin-2 by B16 melanoma cells results in decreased tumour growth and long-term tumour dormancy [J]. Immunology, 2013, 138(3):280-292.
[2] Ercolano, Giuseppe, et al. "Knockdown of PTGS2 by CRISPR/CAS9 system designates a new potential gene target for melanoma treatment." Frontiers in pharmacology 10 (2019): 1456.
[3] Das, Krishna, et al. "Generation of murine tumor cell lines deficient in MHC molecule surface expression using the CRISPR/Cas9 system." PloS one 12.3 (2017): e0174077.
[4] Han, Ping, et al. "Genome-wide CRISPR screening identifies Jak1 deficiency as a mechanism of T-cell resistance." Frontiers in immunology 10 (2019): 251.
[5] Zheng, Yaowu, et al. "Efficient Single Base Editing in Mouse Using Cytosine Base Editor 4." Authorea Preprints (2019).


