Location:Home > Application > Ubigene’s PRDX5 knockout and overexpression cell lines help discover a new mechanismm of castration-resistant prostate cancer progression
Ubigene’s PRDX5 knockout and overexpression cell lines help discover a new mechanismm of castration-resistant prostate cancer progression
Recently, Dr. Rong Wang, from a research group led by Professor Yong Q Chen from Jiangnan University, China, published a research paper titled "Identification of PRDX5 as A Target for The Treatment of Castration-Resistant Prostate Cancer" on Advanced Science (IF=15.1). In this study, CRISPR-Cas9 PRDX5 knockout 22Rv1 cells and LNCaP cells with lentiviral overexpression of PRDX5 gene constructed by Ubigene were used to discover the new mechanism of PRDX5 affecting the progress of castration-resistant prostate cancer (CRPC) by regulating redox, and to explore the therapeutic effect of its targeting new small molecule drug Polaprezinc (POL) on CRPC in cells, animals and clinic. It provides a new target for clinical drug resistance of prostate cancer, and provides the possibility of new drugs combined with abiraterone for patients with CRPC, so as to prolong the life cycle of patients with CRPC.
INTRODUCTION
Prostate cancer (PCa) is a major disease affecting 14.1% of male population worldwide. Charles Huggins' groundbreaking discovery in 1941 established the important role of androgen deprivation therapy (ADT), which can control the development of advanced prostate cancer. Although there is a good response in the early stage, basically all patients using ADT therapy will develop into castration-resistant prostate cancer (CRPC) within 1-3 years of treatment. The state of drug-resistant persistence (DTP) was reported 10 years ago. However, it is not clear whether there are AR inhibitor-related prostate DTP cells.
METHOD AND RESULTS
In order to provide a research basis for solving the problem of lack of treatment in patients with CRPC, the authors explored the important influence of thioredoxin redox pathway on drug-resistant cells in prostate DTP cells by proteomics, and then found the key target PRDX5. In order to confirm the role of PRDX5 in CRPC, the authors overexpressed PRDX5 in LNCaP with relatively low endogenous PRDX5 levels. When the cells were treated with different concentrations of EPI/ENZ, it was found that overexpression of PRDX5 significantly increased the resistance of the cells to EPI and ENZ. Then, PRDX5 was knocked out by CRISPR-Cas9 technology from 22Rv1 with relatively high endogenous PRDX5 level and resistance to ENZ. Knockout of PRDX5 made the cells sensitive to EPI and ENZ. These in vitro data suggest that PRDX5 plays a key role in the resistance of PCa cells to AR inhibitors.
In order to confirm the role of PRDX5 in vivo, the authors obtained PRDX5 knockout mice. The deletion of Prdx5 reduced the survival rate of homozygous mice. In addition, Prdx5 homozygous/MYC transgenic mice could not be obtained. Therefore, the authors determined the effect of partial deletion of Prdx5 on the prostate of MYC transgenic mice. Note that compared with MYCT, Prdx5+/−;MYCT mice significantly delayed the progression of prostatic intraepithelial neoplasia (PIN)/tumor in terms of tissue weight and histopathology. In addition, Prdx5+/−;MYCT mice showed slower resistance to ENZ and significantly inhibited the growth of prostate tumors. The data shows that PRDX5 is very important in the development of CRPC. It is worth noting that knockout of Prdx5 gene alone does not affect prostate weight or prostate histopathology.
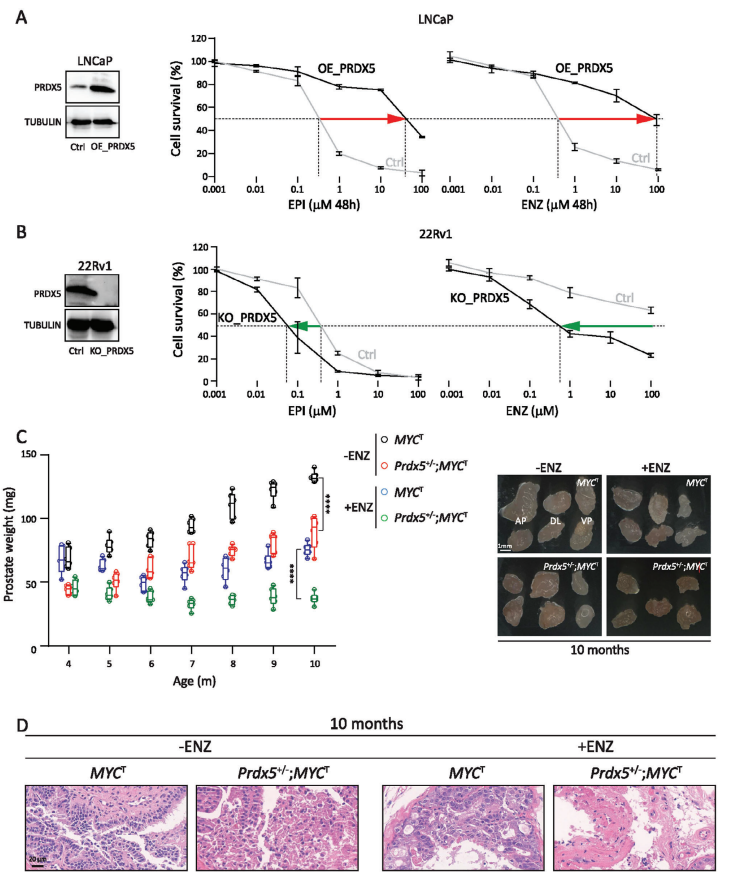
1. The key role of PRDX5 in AR inhibitor resistance and development of CRPC
Then, after verifying the efficacy at the cellular and animal levels, the authors spent two years evaluating the efficacy of PRDX5 small molecule inhibitor Polaprezinc on CRPC at the clinical patient level. The authors recruited 12 patients with CRPC in the Affiliated Hospital of Jiangnan University, and the patients had fully informed consent. These patients were 71-82 years old, with high PSA and Gleason scores at the initial diagnosis, and were diagnosed as CRPC after receiving various drug treatments for many times. Patients 1-8 were treated with abiraterone/prednisone plus POL (75mg b.i.d) for 6 months, and patients 9-12 received other treatments. The PSA level of patients was monitored monthly and the ECT images of patients before and after treatment were took. From the PSA waterfall plot of all patients, it was found that the PSA of patients 1, 7 and 8 decreased significantly during ABI+POL treatment, and the PSA of patients 2, 3, 4 and 5 decreased at some points, but the PSA level was higher after 6 months of treatment, and patient 6 progressed during the treatment. PSA progression occurred in patients 9, 10, 11 and 12 who received other treatments. The average percentage of PSA change in ABI+POL group was 31.31% (95% CI [-41.45%, 104.07%; IQR [207.93]), while that in the other group was 120.69% (95% CI [56.17%, 149.21%; IQR [70.13]). According to the criteria of the Prostate Cancer Clinical Trials Working Group 3 (PCWG3), the authors conducted ECT imaging analysis and found that patients 4, 6, 7 and 8 treated with ABI+POL were all non-progressive diseases (Non-PD), and patients 3 continued to progress (PD). Disease progression (PD) occurred in patients 9 and 12 receiving other treatments. No death related to CRPC occurred in ABI+POL group, while 2 of the 4 patients who received other treatment died of CRPC. These results showed that inhibition of PRDX5 could slow down the progression of PSA (8/8 cases) and stabilize metastatic CRPC (4/5 cases). Other treatments did not stabilize PSA (0/4 cases) or slow down the progress of mCRPC (0/2 cases).
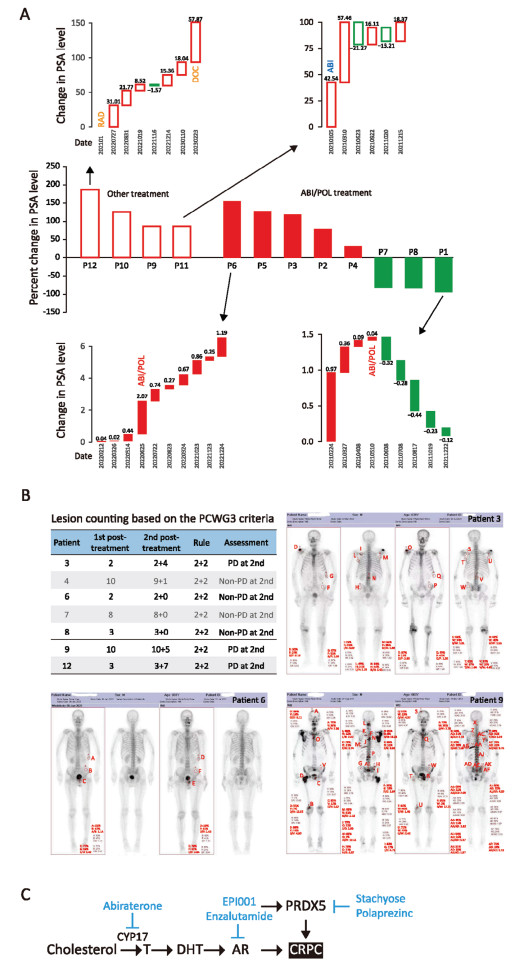
2. The stabilization role of PRDX5 inhibitor Polaprezinc to tumors in CRPC patients
SUMMARY
In this study, the authors demonstrated that AR-blocked drug-resistant DTP cells play an important role in the progress of CRPC mediated by Peroxiredoxin 5 (PRDX5) pathway. In vitro, PRDX5 can improve the resistance of cancer cells to AR inhibitors; In vivo experiments confirmed that it promotes the progress of CRPC. Inhibition of PRDX5 can further inhibit the proliferation of DTP cells, control the development of CRPC in animal models, and stabilize the development of PSA indicators and the progress of metastases in clinical patients. Therefore, this study provides a new mechanism and potential therapeutic target for the treatment of CRPC.
Reference
1. Wang, R., Mi, Y., Ni, J., Wang, Y., Ding, L., Ran, X., Sun, Q., Tan, S.Y., Koeffler, H.P., Feng, N., and Chen, Y.Q. (2023). Identification of PRDX5 as A Target for The Treatment of Castration-Resistant Prostate Cancer. Adv Sci (Weinh), e2304939. 10.1002/advs.202304939.


Ubigene’s PRDX5 knockout and overexpression cell lines help discover a new mechanismm of castration-resistant prostate cancer progression

Recently, Dr. Rong Wang, from a research group led by Professor Yong Q Chen from Jiangnan University, China, published a research paper titled "Identification of PRDX5 as A Target for The Treatment of Castration-Resistant Prostate Cancer" on Advanced Science (IF=15.1). In this study, CRISPR-Cas9 PRDX5 knockout 22Rv1 cells and LNCaP cells with lentiviral overexpression of PRDX5 gene constructed by Ubigene were used to discover the new mechanism of PRDX5 affecting the progress of castration-resistant prostate cancer (CRPC) by regulating redox, and to explore the therapeutic effect of its targeting new small molecule drug Polaprezinc (POL) on CRPC in cells, animals and clinic. It provides a new target for clinical drug resistance of prostate cancer, and provides the possibility of new drugs combined with abiraterone for patients with CRPC, so as to prolong the life cycle of patients with CRPC.
INTRODUCTION
Prostate cancer (PCa) is a major disease affecting 14.1% of male population worldwide. Charles Huggins' groundbreaking discovery in 1941 established the important role of androgen deprivation therapy (ADT), which can control the development of advanced prostate cancer. Although there is a good response in the early stage, basically all patients using ADT therapy will develop into castration-resistant prostate cancer (CRPC) within 1-3 years of treatment. The state of drug-resistant persistence (DTP) was reported 10 years ago. However, it is not clear whether there are AR inhibitor-related prostate DTP cells.
METHOD AND RESULTS
In order to provide a research basis for solving the problem of lack of treatment in patients with CRPC, the authors explored the important influence of thioredoxin redox pathway on drug-resistant cells in prostate DTP cells by proteomics, and then found the key target PRDX5. In order to confirm the role of PRDX5 in CRPC, the authors overexpressed PRDX5 in LNCaP with relatively low endogenous PRDX5 levels. When the cells were treated with different concentrations of EPI/ENZ, it was found that overexpression of PRDX5 significantly increased the resistance of the cells to EPI and ENZ. Then, PRDX5 was knocked out by CRISPR-Cas9 technology from 22Rv1 with relatively high endogenous PRDX5 level and resistance to ENZ. Knockout of PRDX5 made the cells sensitive to EPI and ENZ. These in vitro data suggest that PRDX5 plays a key role in the resistance of PCa cells to AR inhibitors.
In order to confirm the role of PRDX5 in vivo, the authors obtained PRDX5 knockout mice. The deletion of Prdx5 reduced the survival rate of homozygous mice. In addition, Prdx5 homozygous/MYC transgenic mice could not be obtained. Therefore, the authors determined the effect of partial deletion of Prdx5 on the prostate of MYC transgenic mice. Note that compared with MYCT, Prdx5+/−;MYCT mice significantly delayed the progression of prostatic intraepithelial neoplasia (PIN)/tumor in terms of tissue weight and histopathology. In addition, Prdx5+/−;MYCT mice showed slower resistance to ENZ and significantly inhibited the growth of prostate tumors. The data shows that PRDX5 is very important in the development of CRPC. It is worth noting that knockout of Prdx5 gene alone does not affect prostate weight or prostate histopathology.

1. The key role of PRDX5 in AR inhibitor resistance and development of CRPC
Then, after verifying the efficacy at the cellular and animal levels, the authors spent two years evaluating the efficacy of PRDX5 small molecule inhibitor Polaprezinc on CRPC at the clinical patient level. The authors recruited 12 patients with CRPC in the Affiliated Hospital of Jiangnan University, and the patients had fully informed consent. These patients were 71-82 years old, with high PSA and Gleason scores at the initial diagnosis, and were diagnosed as CRPC after receiving various drug treatments for many times. Patients 1-8 were treated with abiraterone/prednisone plus POL (75mg b.i.d) for 6 months, and patients 9-12 received other treatments. The PSA level of patients was monitored monthly and the ECT images of patients before and after treatment were took. From the PSA waterfall plot of all patients, it was found that the PSA of patients 1, 7 and 8 decreased significantly during ABI+POL treatment, and the PSA of patients 2, 3, 4 and 5 decreased at some points, but the PSA level was higher after 6 months of treatment, and patient 6 progressed during the treatment. PSA progression occurred in patients 9, 10, 11 and 12 who received other treatments. The average percentage of PSA change in ABI+POL group was 31.31% (95% CI [-41.45%, 104.07%; IQR [207.93]), while that in the other group was 120.69% (95% CI [56.17%, 149.21%; IQR [70.13]). According to the criteria of the Prostate Cancer Clinical Trials Working Group 3 (PCWG3), the authors conducted ECT imaging analysis and found that patients 4, 6, 7 and 8 treated with ABI+POL were all non-progressive diseases (Non-PD), and patients 3 continued to progress (PD). Disease progression (PD) occurred in patients 9 and 12 receiving other treatments. No death related to CRPC occurred in ABI+POL group, while 2 of the 4 patients who received other treatment died of CRPC. These results showed that inhibition of PRDX5 could slow down the progression of PSA (8/8 cases) and stabilize metastatic CRPC (4/5 cases). Other treatments did not stabilize PSA (0/4 cases) or slow down the progress of mCRPC (0/2 cases).

2. The stabilization role of PRDX5 inhibitor Polaprezinc to tumors in CRPC patients
SUMMARY
In this study, the authors demonstrated that AR-blocked drug-resistant DTP cells play an important role in the progress of CRPC mediated by Peroxiredoxin 5 (PRDX5) pathway. In vitro, PRDX5 can improve the resistance of cancer cells to AR inhibitors; In vivo experiments confirmed that it promotes the progress of CRPC. Inhibition of PRDX5 can further inhibit the proliferation of DTP cells, control the development of CRPC in animal models, and stabilize the development of PSA indicators and the progress of metastases in clinical patients. Therefore, this study provides a new mechanism and potential therapeutic target for the treatment of CRPC.
Reference
1. Wang, R., Mi, Y., Ni, J., Wang, Y., Ding, L., Ran, X., Sun, Q., Tan, S.Y., Koeffler, H.P., Feng, N., and Chen, Y.Q. (2023). Identification of PRDX5 as A Target for The Treatment of Castration-Resistant Prostate Cancer. Adv Sci (Weinh), e2304939. 10.1002/advs.202304939.


