Location:Home > Application > Ubigene KO cells Help Discover A Novel TNFR1 inhibitor - FKC peptide
Ubigene KO cells Help Discover A Novel TNFR1 inhibitor - FKC peptide

Introduction
Tumor necrosis factor (TNF) is an important regulator of inflammatory responses. However, excessive production or abnormal activity of TNF may lead to a series of autoimmune diseases. Current therapies targeting TNF, though effective, have some drawbacks. These treatment methods are expensive and may cause serious side effects. Therefore, specifically targeting its receptor TNFR1 instead of TNF may be a more effective and less side-effect treatment strategy.
Recently, the team led by Chih Hung Lo published a research paper titled “Peptide-based allosteric inhibitor targets TNFR1 conformationally active region and disables receptor-ligand signaling complex” in PNAS (IF=11.1). The study found that FKC, as an allosteric inhibitor, disrupts TNFR1 signaling by binding to TNFR1 and disturbing the receptor's conformational dynamics, rather than by blocking receptor-ligand interactions or disrupting receptor-receptor interactions. This mechanism provides a new perspective for developing novel non-competitive inhibitors and opens up new avenues for specific inhibition of TNFR1. The study used TNFR1 gene knockout HEK293 cells provided by Ubigeneto investigate the specific effects of FKC peptide on the TNFR1 signaling pathway.
FKC peptide interaction with TNFR1 and receptor conformational changes
Researchers found that FKC peptide (FKCRRWQWRMKK) can interact with TNFR1 and induce conformational changes in the receptor. Using a FRET (Fluorescence Resonance Energy Transfer) biosensor for TNFR1, researchers observed that FKC peptide dose-dependently reduced FRET efficiency, indicating that FKC altered the conformational state of TNFR1. FKC peptide had no effect on TNFR2 isotypes, demonstrating the specificity of FKC peptide's interaction with TNFR1.
Specific inhibition of TNFR1 signaling by FKC peptide
FKC peptide dose-dependently inhibited TNF-induced IκBα phosphorylation, degradation, and NF-κB component p65 phosphorylation in HEK293 cells. These are key steps in the TNFR1 signaling pathway. Using luciferase reporter gene analysis, FKC peptide inhibited TNF-induced NF-κB activation with an IC50 of 27 µM. In TNFR1 gene knockout HEK293 cells (constructed by Ubigene), FKC peptide did not affect basal NF-κB activity, confirming the specific inhibitory effect of FKC peptide on the TNFR1 signaling pathway.
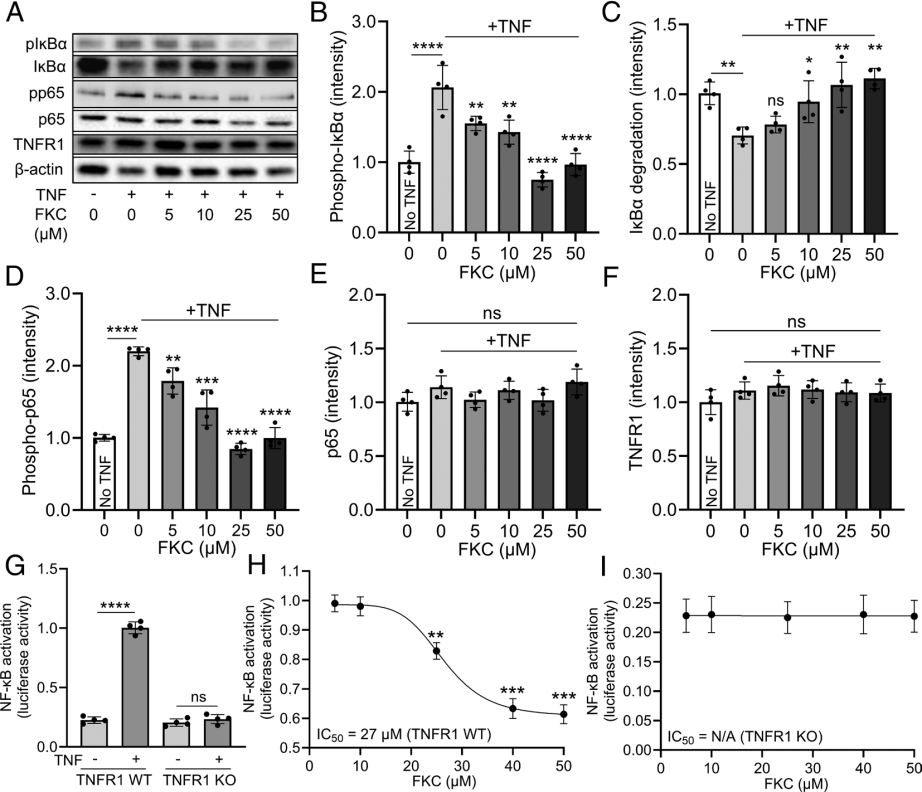
Figure 1 FKC peptide specifically inhibits the TNFR1 signaling pathway.
Effects of FKC peptide on reducing inflammation in vivo
Researchers used a mouse model of intraperitoneal TNF injection to simulate an inflammatory response and evaluate the effects of FKC peptide. FKC peptide treatment can significantly reduce the levels of various inflammatory factors induced by TNF (such as TNF, MCP-1, IFN-γ, IL-1α, IL-1β, and IL-6) in mouse plasma. FKC peptide reduced the number of CD68-positive cells in the livers, kidneys, and lungs of mice induced by TNF, indicating a reduction in macrophage activation.
FKC peptide targets the conformationally active region of TNFR1 and disrupts receptor dynamics
Researchers used computational methods to determine the binding site of FKC peptide to TNFR1 and found that FKC peptide stably bound near the CRD2/3 of the TNFR1 dimer, which is the receptor's conformationally active region. The binding of FKC peptide reduced the dynamic changes of TNFR1's CRD2/3 and CRD4 domains, leading to the receptor adopting an open and inactive conformation, hindering the recruitment of downstream signaling molecules.
Allosteric inhibition mechanism of FKC peptide
FKC peptide, as an allosteric inhibitor, acts but not by blocking ligand binding or disrupting receptor-receptor interactions. FKC peptide can even prevent other small molecules (such as Zafirlukast) from disrupting TNFR1 receptor-receptor interactions.
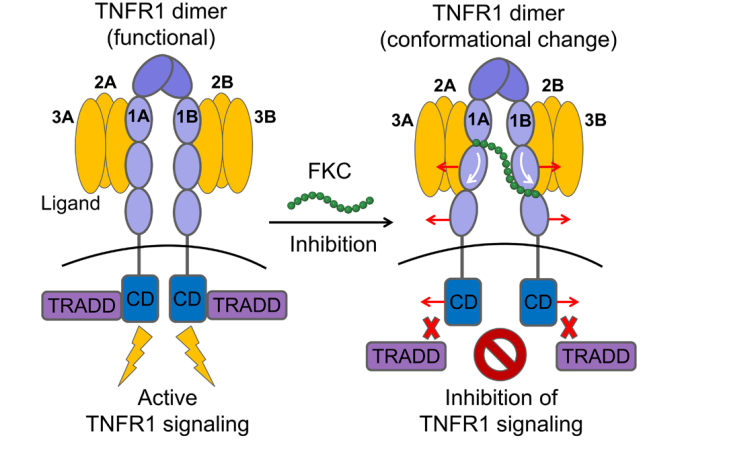
Figure 2 Mechanism diagram
Conclusion
The research results demonstrate that FKC peptide selectively targets the conformationally active region of TNFR1, non-competitively inhibiting TNFR1 signaling and reducing inflammatory responses. This study provides feasibility evidence for targeted therapy against the conformationally active region of TNFR1 and opens up new directions for the development of more effective and cost-effective TNFR1 inhibitors.
References
Zeng, Jialiu et al. Peptide-based allosteric inhibitor targets TNFR1 conformationally active region and disables receptor-ligand signaling complex. Proceedings of the National Academy of Sciences of the United States of America vol. 121,14 (2024): e2308132121.

Ubigene KO cells Help Discover A Novel TNFR1 inhibitor - FKC peptide

Introduction
Tumor necrosis factor (TNF) is an important regulator of inflammatory responses. However, excessive production or abnormal activity of TNF may lead to a series of autoimmune diseases. Current therapies targeting TNF, though effective, have some drawbacks. These treatment methods are expensive and may cause serious side effects. Therefore, specifically targeting its receptor TNFR1 instead of TNF may be a more effective and less side-effect treatment strategy.
Recently, the team led by Chih Hung Lo published a research paper titled “Peptide-based allosteric inhibitor targets TNFR1 conformationally active region and disables receptor-ligand signaling complex” in PNAS (IF=11.1). The study found that FKC, as an allosteric inhibitor, disrupts TNFR1 signaling by binding to TNFR1 and disturbing the receptor's conformational dynamics, rather than by blocking receptor-ligand interactions or disrupting receptor-receptor interactions. This mechanism provides a new perspective for developing novel non-competitive inhibitors and opens up new avenues for specific inhibition of TNFR1. The study used TNFR1 gene knockout HEK293 cells provided by Ubigeneto investigate the specific effects of FKC peptide on the TNFR1 signaling pathway.
FKC peptide interaction with TNFR1 and receptor conformational changes
Researchers found that FKC peptide (FKCRRWQWRMKK) can interact with TNFR1 and induce conformational changes in the receptor. Using a FRET (Fluorescence Resonance Energy Transfer) biosensor for TNFR1, researchers observed that FKC peptide dose-dependently reduced FRET efficiency, indicating that FKC altered the conformational state of TNFR1. FKC peptide had no effect on TNFR2 isotypes, demonstrating the specificity of FKC peptide's interaction with TNFR1.
Specific inhibition of TNFR1 signaling by FKC peptide
FKC peptide dose-dependently inhibited TNF-induced IκBα phosphorylation, degradation, and NF-κB component p65 phosphorylation in HEK293 cells. These are key steps in the TNFR1 signaling pathway. Using luciferase reporter gene analysis, FKC peptide inhibited TNF-induced NF-κB activation with an IC50 of 27 µM. In TNFR1 gene knockout HEK293 cells (constructed by Ubigene), FKC peptide did not affect basal NF-κB activity, confirming the specific inhibitory effect of FKC peptide on the TNFR1 signaling pathway.

Figure 1 FKC peptide specifically inhibits the TNFR1 signaling pathway.
Effects of FKC peptide on reducing inflammation in vivo
Researchers used a mouse model of intraperitoneal TNF injection to simulate an inflammatory response and evaluate the effects of FKC peptide. FKC peptide treatment can significantly reduce the levels of various inflammatory factors induced by TNF (such as TNF, MCP-1, IFN-γ, IL-1α, IL-1β, and IL-6) in mouse plasma. FKC peptide reduced the number of CD68-positive cells in the livers, kidneys, and lungs of mice induced by TNF, indicating a reduction in macrophage activation.
FKC peptide targets the conformationally active region of TNFR1 and disrupts receptor dynamics
Researchers used computational methods to determine the binding site of FKC peptide to TNFR1 and found that FKC peptide stably bound near the CRD2/3 of the TNFR1 dimer, which is the receptor's conformationally active region. The binding of FKC peptide reduced the dynamic changes of TNFR1's CRD2/3 and CRD4 domains, leading to the receptor adopting an open and inactive conformation, hindering the recruitment of downstream signaling molecules.
Allosteric inhibition mechanism of FKC peptide
FKC peptide, as an allosteric inhibitor, acts but not by blocking ligand binding or disrupting receptor-receptor interactions. FKC peptide can even prevent other small molecules (such as Zafirlukast) from disrupting TNFR1 receptor-receptor interactions.

Figure 2 Mechanism diagram
Conclusion
The research results demonstrate that FKC peptide selectively targets the conformationally active region of TNFR1, non-competitively inhibiting TNFR1 signaling and reducing inflammatory responses. This study provides feasibility evidence for targeted therapy against the conformationally active region of TNFR1 and opens up new directions for the development of more effective and cost-effective TNFR1 inhibitors.
References
Zeng, Jialiu et al. Peptide-based allosteric inhibitor targets TNFR1 conformationally active region and disables receptor-ligand signaling complex. Proceedings of the National Academy of Sciences of the United States of America vol. 121,14 (2024): e2308132121.

