

Location:Home > Application > IF=27.7 | Ubigene KO Cells Help Reveal New Functions & Mechanism of Functional Oligosaccharides(FOs)
IF=27.7 | Ubigene KO Cells Help Reveal New Functions & Mechanismof Functional Oligosaccharides(FOs)

Recently, a research team led by Professors Ting Li and Xingbin Yang from Shaanxi Normal University published a paper titled “Nondigestible stachyose binds membranous HSP90β on small intestinal epithelium to regulate the exosomal miRNAs: a new function & mechanism” in Cell Metabolism. This study reveals for the first time the new nutritional functions and mechanisms of functional oligosaccharides(FOs) in the upper gastrointestinal (GI) tract. The research utilized HSP90β knockout Mode-K small intestinal epithelial cells constructed by Ubigene to investigate the effects of stachyose on the exosomal miRNA of KO cells, providing in-depth insight into how stachyose, which is not absorbed in the upper gastrointestinal (GI) tract , regulates the composition of small intestinal exosomal miRNAs by directly interacting with the HSP90β on the membrane of small intestinal epithelial cells, thereby demonstrating a new nutritional function.
For a long time, it has been generally believed that functional oligosaccharides do not interact with the proximal small intestine; they are not digested or absorbed by the upper GI tract but instead recognized as “passersby” in the small intestine, reaching the distal colon directly, where they promote the proliferation of beneficial bacteria, thereby playing the classic nutritional role of prebiotics. Surprisingly, this research team utilized techniques such as pull-down assay and CRISPR/Cas9 and discover that stachyose accumulates significantly on the surface of the cell membrane and binds to the heat shock protein HSP90β present on the small intestinal epithelial cell membrane although it does not enter epithelial cells of the small intestine for the first time.
HSP90 is described as a regulatory switch for exosome secretion, while exosomes serve as important messengers for intercellular communication, transporting miRNAs, proteins, and other signaling molecules to recipient cells, activating intracellular signaling pathways and regulating physiological functions in the body.
The team further revealed that stachyose reconstitutes the profile of exosomal miRNAs and exerts a novel function by directly acting on the HSP90β of small intestinal epithelial cell membranes with fecal miRNA transplantation and Small RNA-16S rRNA sequencing. They were the first to elucidate this new mechanism by which ,FOs such as stachyose, regulate gut microbiota through this novel function, leading to indirect prebiotic effects.
This discovery challenges the inherent belief that non-digestible oligosaccharides have no communication with the proximal small intestine, expanding the theoretical framework of the nutritional spectrum and mechanisms of classic prebiotic oligosaccharides. It provides new perspectives and insights for exploring the hidden nutritional functions driven by functional oligosaccharides in the upper gastrointestinal tract.
1. Stachyose, a type of oligosaccharides that is not absorbed by the upper gastrointestinal tract and does not get absorbed by the intestinal epithelial cells, notably accumulates on the surface of cell membranes. Further more, it was found that oligosaccharides can directly and stably bind to the HSP90β protein with pull-down assays, protein mass spectrometry, and molecular dynamics simulations.
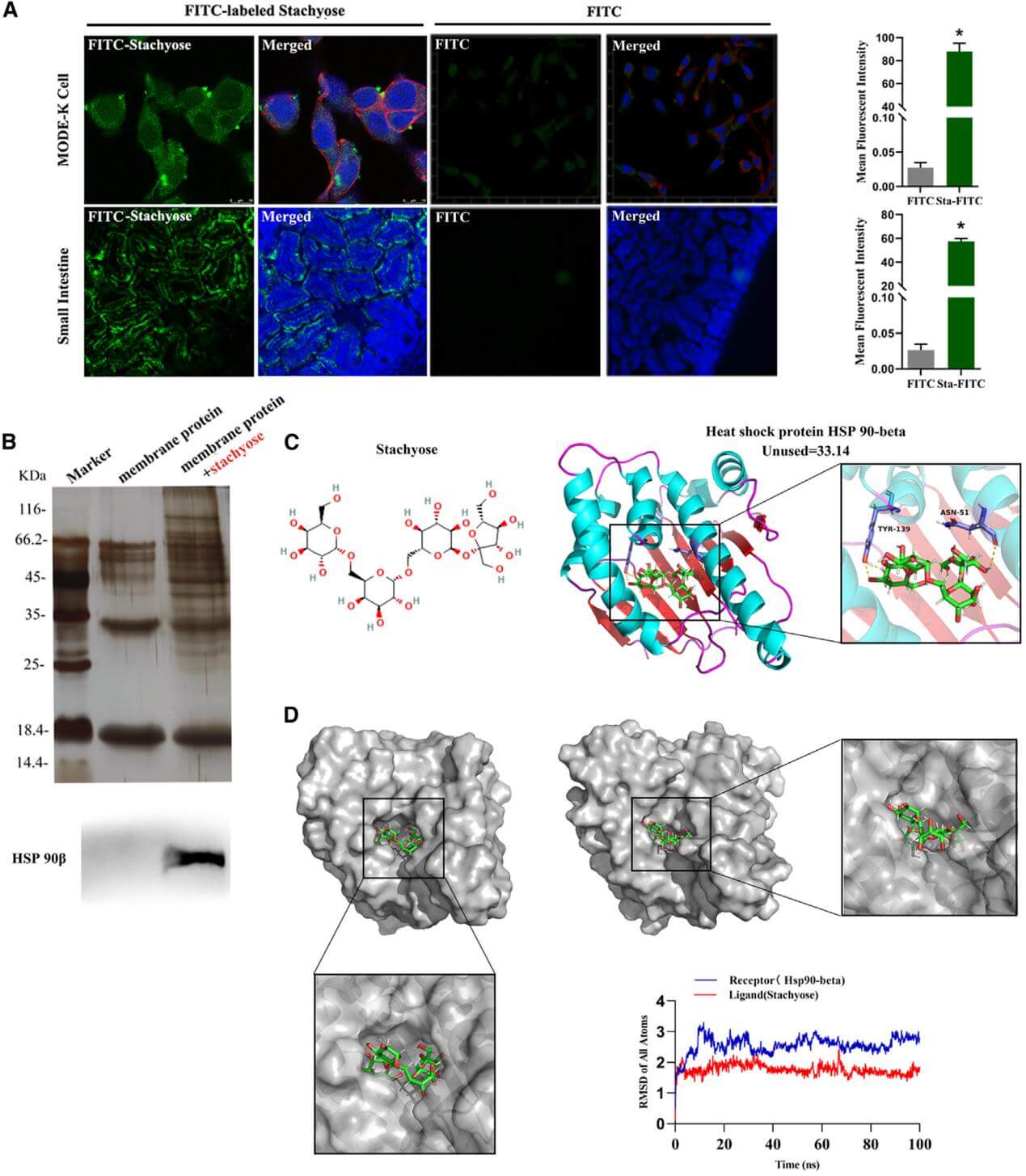
Figure 1.Stachyose directly interacts with HSP90β, which is located at the membrane of mouse small intestinal epithelial cells
2. Stachyose significantly alters the profile of exosomal miRNAs secreted by intestinal epithelial cells. However, when the HSP90β gene in intestinal epithelial cells was knocked out by CRISPR-Cas9 (constructed by Ubigene), the accumulation of Stachyose on the membrane of intestinal epithelial cells and its regulatory effect on intestinal exosomal miRNAs essentially disappear.
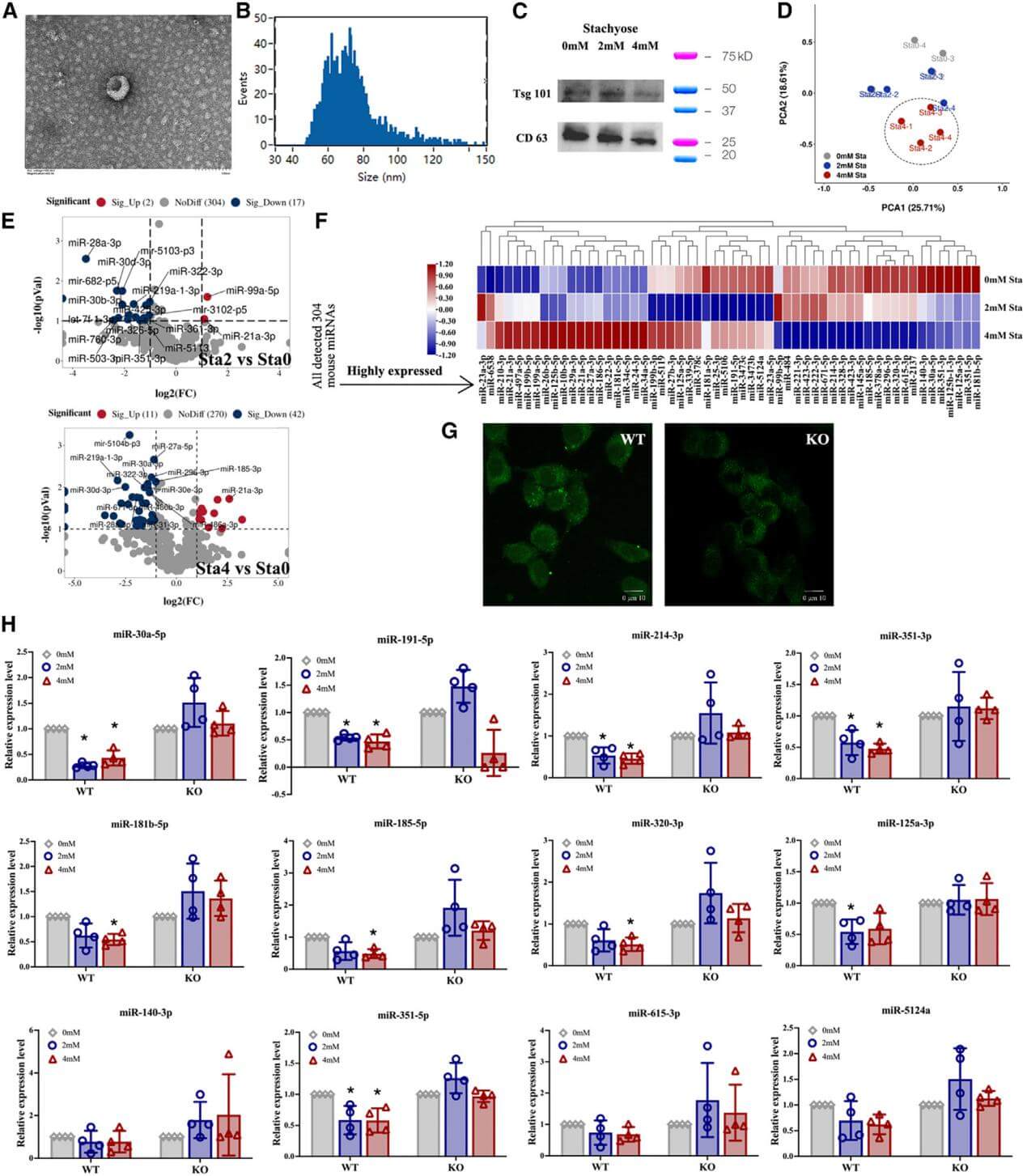
Figure 2. Knockout of HSP90β in MODE-K cells eliminates the accumulation of stachyose on the cell membrane and the regulatory effects of stachyose on the exosomal miRNA expression
3. Stachyosesignificantly alters the profile of exosomal miRNAs secreted by intestinal cells in the feces of both mice and humans, and the regulatory effect of Stachyoseon intestinal exosomal miRNAs is independent of the gut microbiota.
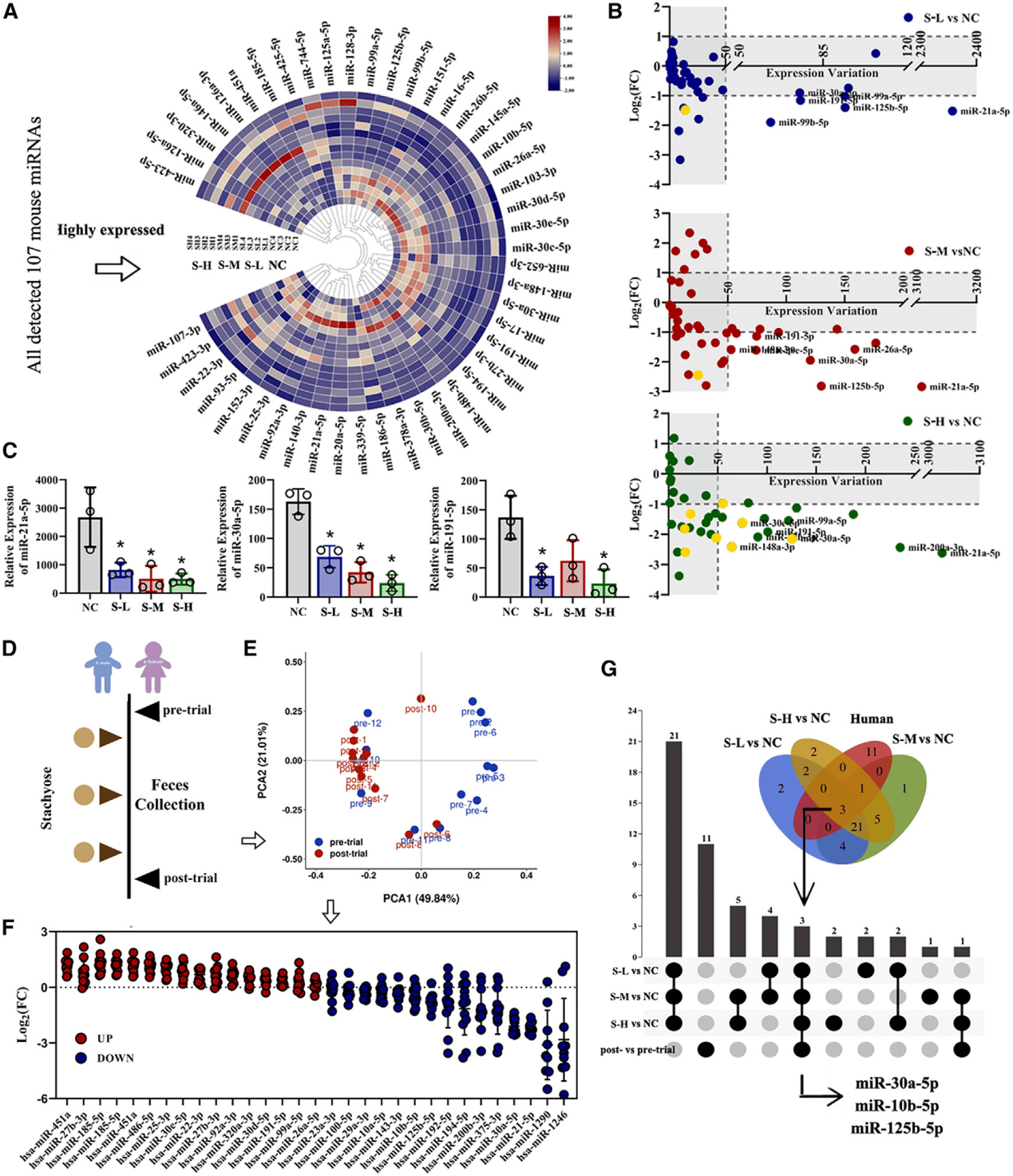
Figure 3. Stachyose reconstitutes fecal miRNA profiles in mice and humans
4. Stachyose alters the exosomal miRNAs in the gut, further reshaping the gut microbiota structure of mice, particularly increasing the abundance of Lactobacillus in the intestines.
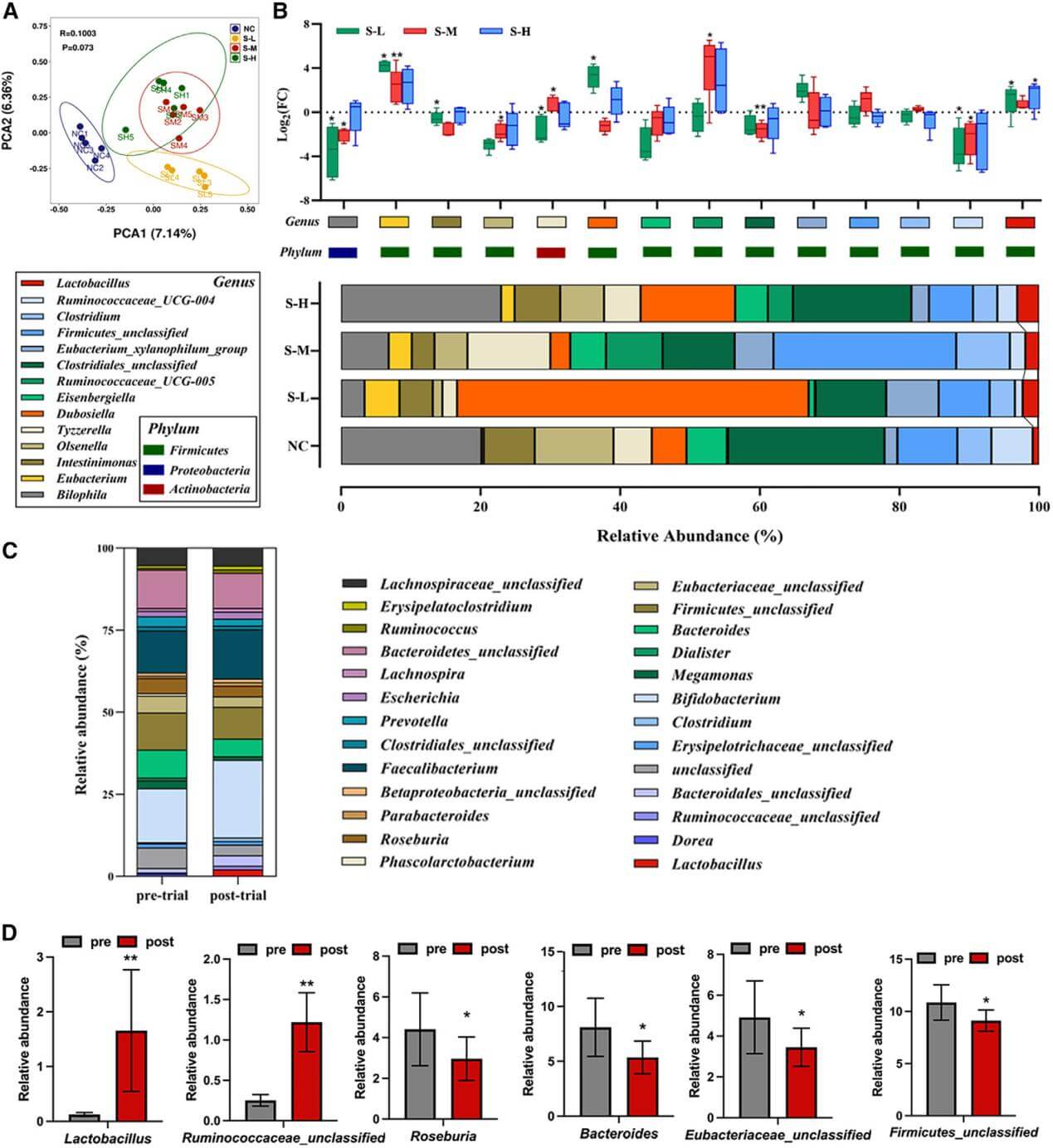
Figure 4. Stachyose shapes the gut microbiota in mice and human
5. Stachyose significantly downregulates the miRNA miR-30a-5p secreted by small intestinal cells into the gut lumen, while miR-30a-5p can specifically inhibit the growth of Lactobacillus reuteri.
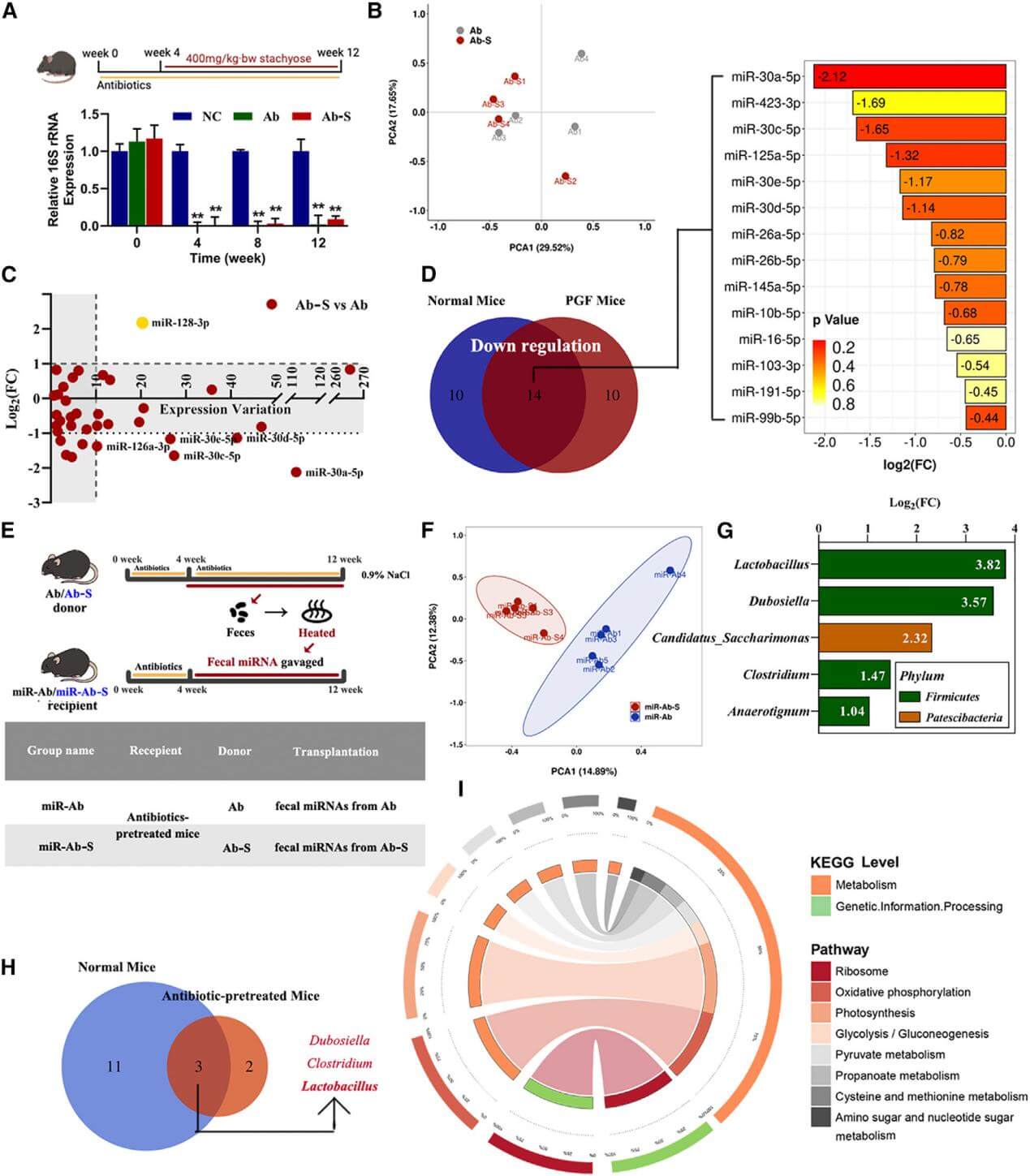
Figure 5. The axis of stachyose-fecal miRNA-gut microbiota
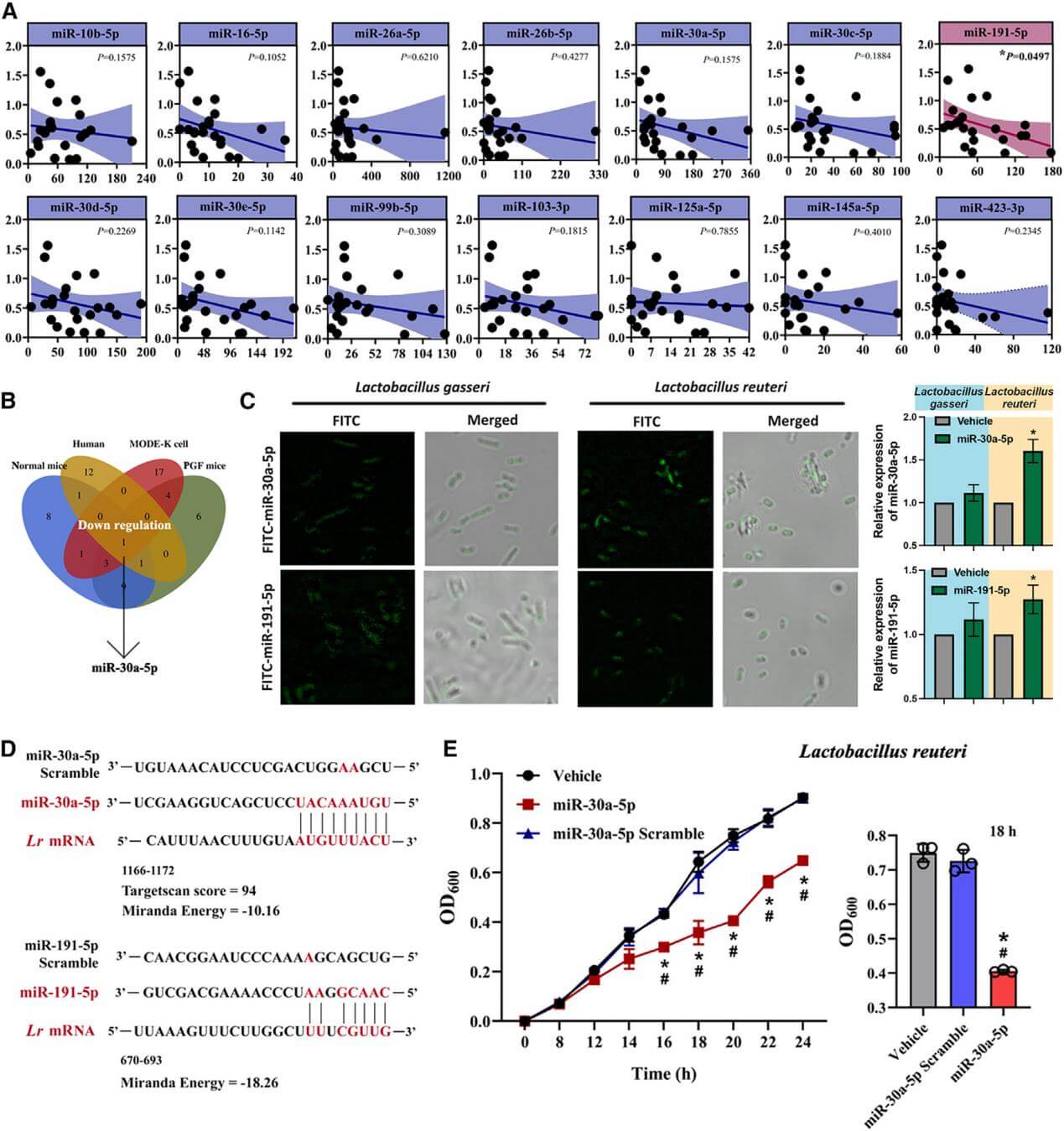
Figure 6. miR-30a-5p restrains the proliferation of Lactobacillus reuteri
Ubigene’s 4500+ KO cells off-shelf, inquire now with your gene of interest!
Reference
Li, Ting, et al. "Nondigestible stachyose binds membranous HSP90β on small intestinal epithelium to regulate the exosomal miRNAs: A new function and mechanism." Cell Metabolism (2024). https://doi.org/10.1016/j.cmet.2024.10.012


IF=27.7 | Ubigene KO Cells Help Reveal New Functions & Mechanismof Functional Oligosaccharides(FOs)

Recently, a research team led by Professors Ting Li and Xingbin Yang from Shaanxi Normal University published a paper titled “Nondigestible stachyose binds membranous HSP90β on small intestinal epithelium to regulate the exosomal miRNAs: a new function & mechanism” in Cell Metabolism. This study reveals for the first time the new nutritional functions and mechanisms of functional oligosaccharides(FOs) in the upper gastrointestinal (GI) tract. The research utilized HSP90β knockout Mode-K small intestinal epithelial cells constructed by Ubigene to investigate the effects of stachyose on the exosomal miRNA of KO cells, providing in-depth insight into how stachyose, which is not absorbed in the upper gastrointestinal (GI) tract , regulates the composition of small intestinal exosomal miRNAs by directly interacting with the HSP90β on the membrane of small intestinal epithelial cells, thereby demonstrating a new nutritional function.

For a long time, it has been generally believed that functional oligosaccharides do not interact with the proximal small intestine; they are not digested or absorbed by the upper GI tract but instead recognized as “passersby” in the small intestine, reaching the distal colon directly, where they promote the proliferation of beneficial bacteria, thereby playing the classic nutritional role of prebiotics. Surprisingly, this research team utilized techniques such as pull-down assay and CRISPR/Cas9 and discover that stachyose accumulates significantly on the surface of the cell membrane and binds to the heat shock protein HSP90β present on the small intestinal epithelial cell membrane although it does not enter epithelial cells of the small intestine for the first time.
HSP90 is described as a regulatory switch for exosome secretion, while exosomes serve as important messengers for intercellular communication, transporting miRNAs, proteins, and other signaling molecules to recipient cells, activating intracellular signaling pathways and regulating physiological functions in the body.
The team further revealed that stachyose reconstitutes the profile of exosomal miRNAs and exerts a novel function by directly acting on the HSP90β of small intestinal epithelial cell membranes with fecal miRNA transplantation and Small RNA-16S rRNA sequencing. They were the first to elucidate this new mechanism by which ,FOs such as stachyose, regulate gut microbiota through this novel function, leading to indirect prebiotic effects.
This discovery challenges the inherent belief that non-digestible oligosaccharides have no communication with the proximal small intestine, expanding the theoretical framework of the nutritional spectrum and mechanisms of classic prebiotic oligosaccharides. It provides new perspectives and insights for exploring the hidden nutritional functions driven by functional oligosaccharides in the upper gastrointestinal tract.
1. Stachyose, a type of oligosaccharides that is not absorbed by the upper gastrointestinal tract and does not get absorbed by the intestinal epithelial cells, notably accumulates on the surface of cell membranes. Further more, it was found that oligosaccharides can directly and stably bind to the HSP90β protein with pull-down assays, protein mass spectrometry, and molecular dynamics simulations.

Figure 1.Stachyose directly interacts with HSP90β, which is located at the membrane of mouse small intestinal epithelial cells
2. Stachyose significantly alters the profile of exosomal miRNAs secreted by intestinal epithelial cells. However, when the HSP90β gene in intestinal epithelial cells was knocked out by CRISPR-Cas9 (constructed by Ubigene), the accumulation of Stachyose on the membrane of intestinal epithelial cells and its regulatory effect on intestinal exosomal miRNAs essentially disappear.

Figure 2. Knockout of HSP90β in MODE-K cells eliminates the accumulation of stachyose on the cell membrane and the regulatory effects of stachyose on the exosomal miRNA expression
3. Stachyosesignificantly alters the profile of exosomal miRNAs secreted by intestinal cells in the feces of both mice and humans, and the regulatory effect of Stachyoseon intestinal exosomal miRNAs is independent of the gut microbiota.

Figure 3. Stachyose reconstitutes fecal miRNA profiles in mice and humans
4. Stachyose alters the exosomal miRNAs in the gut, further reshaping the gut microbiota structure of mice, particularly increasing the abundance of Lactobacillus in the intestines.

Figure 4. Stachyose shapes the gut microbiota in mice and human
5. Stachyose significantly downregulates the miRNA miR-30a-5p secreted by small intestinal cells into the gut lumen, while miR-30a-5p can specifically inhibit the growth of Lactobacillus reuteri.

Figure 5. The axis of stachyose-fecal miRNA-gut microbiota

Figure 6. miR-30a-5p restrains the proliferation of Lactobacillus reuteri
Ubigene’s 4500+ KO cells off-shelf, inquire now with your gene of interest!
Reference
Li, Ting, et al. "Nondigestible stachyose binds membranous HSP90β on small intestinal epithelium to regulate the exosomal miRNAs: A new function and mechanism." Cell Metabolism (2024). https://doi.org/10.1016/j.cmet.2024.10.012


