Discovering Common Cell Models Used in Lung Cancer Research!

Discovering Common Cell Models Used in Lung Cancer Research!
Lung cancer is one of the most common cancers worldwide in terms of incidence and mortality rates. According to its histopathological characteristics, it is mainly divided into non-small cell lung cancer (including adenocarcinoma and squamous cell carcinoma, which account for 84% of all lung cancers) and small cell lung cancer. To better understand the development of lung cancer and explore effective treatment options, researchers usually utilize the lung cancer cell models to simulate the biological characteristics of lung cancer in vitro, providing essential scientific evidence for the diagnosis and treatment of the disease. Today, we will introduce several common cell models used in lung cancer research and their applications.
Human Non-Small Cell Lung Cancer Cell Line (A549)
Catalog: YC-C016
Origin: Human lung adenocarcinoma cell line derived from lung adenocarcinoma tissue of a 58-year-old male
Genetic Mutation: KRAS mutation (G12S)
Characteristics: This cell line secretes pulmonary surfactant and exhibits type II alveolar epithelial cell functions, making it a classic model for lung adenocarcinoma and type II alveolar epithelial cells[1]. It is widely used in anticancer drug screening and evaluation, lung cancer tumor and gene editing and other research.
Application:
The UXS1 gene may play an important role in cellular metabolism processes. Doshi et al.[2] used UXS1-iKO (inducible type) A549 cells and UXS1-KO A549 cells, discovering that the deletion of the UXS1 gene disrupts sugar nucleotide clearance. Compared to normal A549 cells, UXS1 knockout cells show a significant decrease in proliferation capabilities under specific metabolic conditions, indicating that sugar nucleotide metabolism is critical in maintaining the proliferation advantage of cancer cells. Further studies revealed that alterations in sugar nucleotide metabolism also affect signaling pathways in cancer cells. Some signals related to cell survival and drug resistance were interfered with, suggesting that regulating nucleotide sugar metabolism may help overcome cancer cell resistance, providing new ideas for developing novel anticancer drugs and combination therapies.
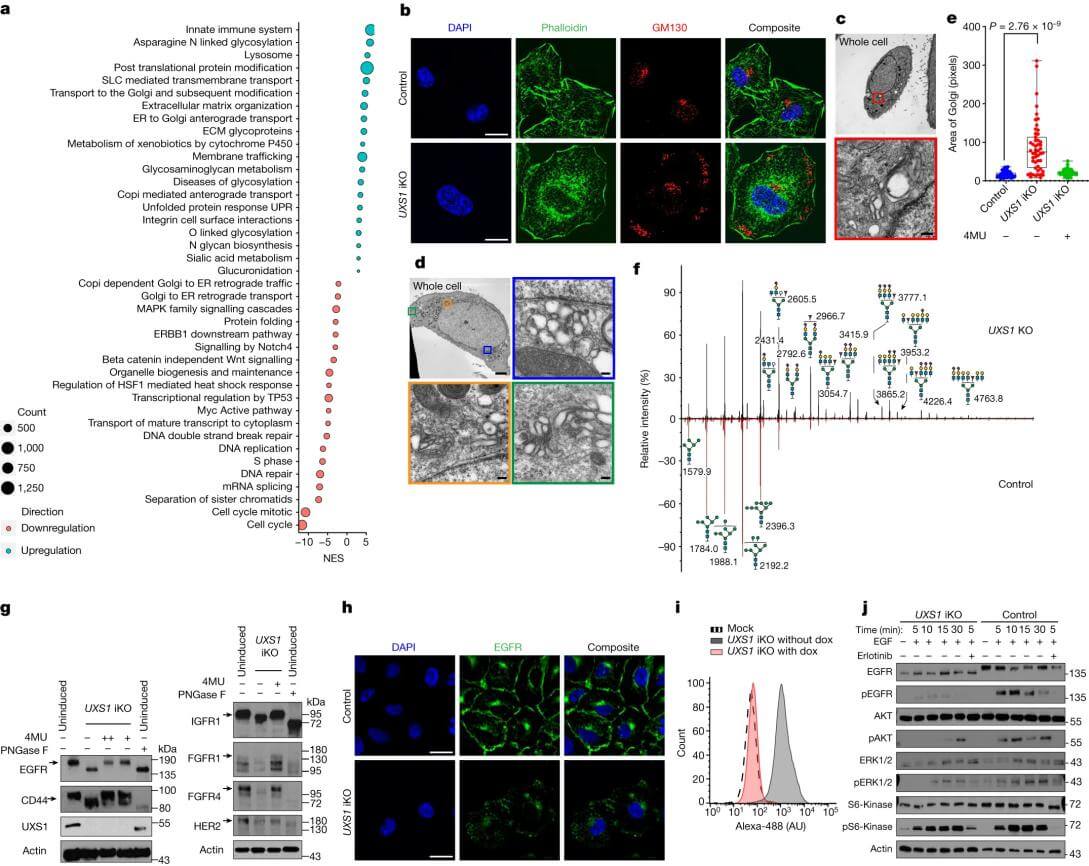
Figure 1. Accumulation of UDPGA leads to Golgi dysfunction, resulting in EGFR inactivation
Explore lung cancer cells and more KO cell lines >>>
Human Non-Small Cell Lung Cancer Cell Line (NCI-H1299)
Catalog: YC-D010
Origin: This cell line was established from a lymph node metastasis in a patient who had undergone radiotherapy, classified as a large cell carcinoma subtype of non-small cell lung cancer
Genetic Mutation: Loss of function of TP53[3]
Characteristics: The loss of TP53 leads to abnormal responses and repair capabilities for DNA damage. It has strong proliferation, migration, and invasion abilities. Commonly used in research on metastasis mechanisms and tumor invasion, TP53-related signaling pathways, drug resistance, and targeted therapies.
Application:
Yan et al.[4] used LAPTM4B knockout NCI-H1299 cell models to study the effect of LAPTM4B on ferroptosis in non-small cell lung cancer. The results revealed that knocking out LAPTM4B significantly alters metabolites associated with ferroptosis, particularly those related to glutathione, cysteine, and methionine metabolism. The loss of LAPTM4B resulted in increased lipid peroxidation and malondialdehyde (MDA) levels while reducing glutathione (GSH) levels, which are characteristic features of ferroptosis. Additionally, LAPTM4B knockout caused structural abnormalities in mitochondria, manifested as reduced size and deformation of mitochondria. These results indicate that LAPTM4B inhibits the ubiquitin-proteasome degradation of SLC7A11 protein to suppress ferroptosis, thereby promoting the progression of NSCLC.
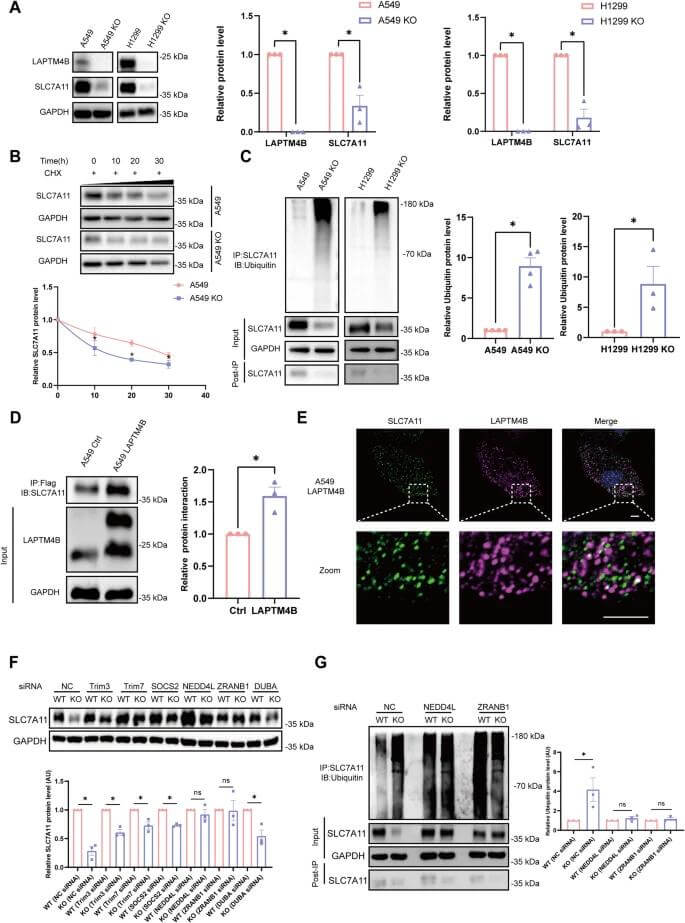
Figure 2. LAPTM4B inhibits the ubiquitination-proteasomal degradation of SLC7A11
Human Lung Adenocarcinoma Cell Line (PC9)
Catalog: YC-C114
Origin: Lung adenocarcinoma (well-differentiated) tissue
Genetic Mutation: EGFR mutation (exon 19 deletion, del E746_A750)
Characteristics: The EGFR mutation makes it highly sensitive to EGFR inhibitors, and it serves as a classic model for targeted therapy research in lung adenocarcinoma, especially for studies on EGFR-TKIs (such as erlotinib and osimertinib) resistance[5]. Additionally, it is often used in research on tumor proliferation and invasion mechanisms.
Application:
Pfeifer et al.[6] used PC9 cells for whole-genome CRISPR knockout (CRISPRn) and activation (CRISPRa) screening to identify driving factors for resistance to EGFR inhibitors in EGFR-mutant lung cancer. To validate these resistance genes, the researchers constructed cell models with gene knockout or overexpression. Among these, knockout of the NF2 gene resulted in PC9 cell resistance to osimertinib and activated the Hippo signaling pathway, specifically the YAP1/WWTR1/TEAD axis. This activation was accompanied by increased nuclear localization of YAP1 and WWTR1 as well as enhanced TEAD reporter gene activity. Moreover, inhibiting the transcriptional activity of the YAP1/WWTR1/TEAD axis could resensitize resistant cells to osimertinib and prevent the formation of persistent resistant cells. These findings indicate that the Hippo signaling pathway plays a crucial role in the resistance of EGFR-mutant lung cancer, providing new therapeutic targets to overcome resistance.
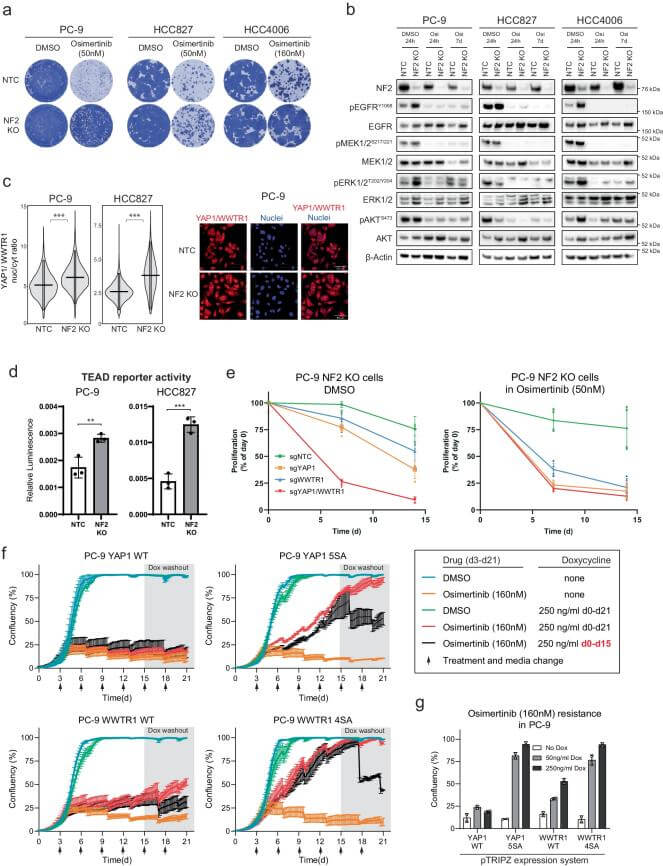
Figure 3. Hippo pathway modulates osimertinib response in EGFR mutant lung cancer
In addition to the commonly used lung cancer cells mentioned above, there are other types of lung cancer cells such as NCI-H520 (human lung squamous carcinoma cells), NCI-H1688 (human small cell lung cancer cells), LLC (mouse lung cancer cells), etc. Their unique origins, genetic mutation characteristics, resistances, and metastatic abilities make them important tools in lung cancer research. Researchers can choose the appropriate cell lines based on their research objectives to effectively advance both basic and clinical studies on lung cancer.
Ubigene can provide gene editing (KO/KI/PM) and stable cell line customization services for lung cancer research-related cells. Please feel free to consult us!
Figure 4. Gene Editing Experiment Images (selected)
References
[1] Foster, Kimberly A., et al. "Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism." Experimental cell research 243.2 (1998): 359-366.
[2] Doshi, M.B., Lee, N., Tseyang, T. et al. Disruption of sugar nucleotide clearance is a therapeutic vulnerability of cancer cells. Nature 623, 625–632 (2023). https://doi.org/10.1038/s41586-023-06676-3
[3] Maj, Ewa, et al. "Differential response of lung cancer cell lines to vitamin D derivatives depending on EGFR, KRAS, p53 mutation status and VDR polymorphism." The Journal of steroid biochemistry and molecular biology 193 (2019): 105431.
[4] Yan, R., Liu, D., Guo, H. et al. LAPTM4B counteracts ferroptosis via suppressing the ubiquitin-proteasome degradation of SLC7A11 in non-small cell lung cancer. Cell Death Dis 15, 436 (2024). https://doi.org/10.1038/s41419-024-06836-x
[5] Ohara, Shuta, Kenichi Suda, and Tetsuya Mitsudomi. "Cell Line Models for Acquired Resistance to First-Line Osimertinib in Lung Cancers—Applications and Limitations." Cells 10.2 (2021): 354.
[6] Pfeifer, M., Brammeld, J.S., Price, S. et al. Genome-wide CRISPR screens identify the YAP/TEAD axis as a driver of persister cells in EGFR mutant lung cancer. Commun Biol 7, 497 (2024). https://doi.org/10.1038/s42003-024-06190-w


