

Location:Home > Application > What are the commonly used cell lines for prostate cancer research?
What are the commonly used cell lines for prostate cancer research?

Prostate cancer is one of the most common cancers in men, accounting for 26% of all diagnosed cases globally and 11% of estimated cancer-related deaths[1]. Currently, the standard treatment for prostate cancer is androgen receptor (AR) deprivation. However, when the disease progresses to the metastatic stage, it becomes ineffective due to androgen resistance, and acquired resistance remains a major issue. Prostate cancer cell models play an important role in prostate cancer research, which can be used to explore the pathogenesis, study cell biology characteristics, conduct drug screening and mechanism of action research, and discover new prostate cancer biomarkers. Next, let’s learn how three commonly used human prostate cancer cell lines provide strong support for prostate cancer research.
Catalog#: YC-C009
Characteristics: The cells exhibit slight adherent growth characteristics and are tumorigenic in nude mice. They respond to 5-alpha-dihydrotestosterone (growth regulators and acid phosphatase products) and express human prostate acid phosphatase, AR (androgen receptor, a marker for glandular formation and luminal differentiation), and PSA (prostate-specific antigen). Their growth is inhibited by androgen withdrawal, resembling human prostate adenocarcinoma. Neuroendocrine markers and stem cell-related marker CD44 are negative[2].
Application:
To identify the causes of enzalutamide sensitivity or resistance in prostate cancer treatment, the authors performed Genome-wide CRISPR knockout screen using LNCaP cells[1]. They discovered that key genes involved in epigenetic regulation and stem cell biology contribute to enzalutamide resistance. They also found that HOXA9 deletion increased enzalutamide sensitivity, while its overexpression caused resistance. Further experiments validated that HOXA9 inhibitors enhanced enzalutamide effectiveness and restored the response to enzalutamide in RB-p53 double-mutant cells. These findings suggest that inhibiting HOXA9 could be a therapeutic strategy for treating enzalutamide-resistant prostate cancer[3].
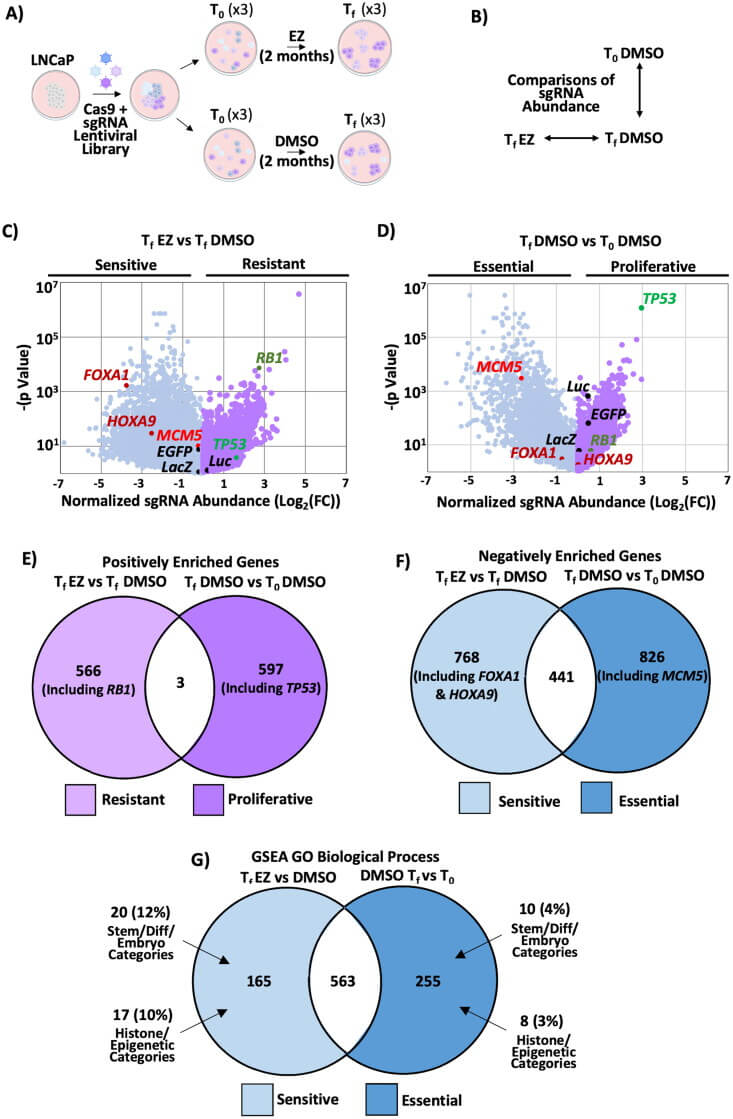
Figure 1: Genome-wide CRISPR knockout screening reveals that stem cell and epigenetic-related genes are crucial for cell viability under enzalutamide treatment
KO cells in stock - Prostate cancer cells and more KO cells, contact us!
Catalog#:YC-C010
Origin: Derived from a 62-year-old Caucasian male with stage IV prostate adenocarcinoma bone metastasis.
Characteristics: The cells exhibit low levels of acid phosphatase and 5-alpha-reductase activities, do not express AR (androgen receptor) or PSA (prostate-specific antigen), and their proliferation is independent of androgens, resembling small cell neuroendocrine carcinoma (SCNC) of the prostate. Neuroendocrine markers and stem cell-related marker CD44 are positive (+)[2].
Application:
To design new PC treatment interventions, CRISPR/Cas9-mediated homologous recombination gene editing was applied to perform site-specific elimination in PC-3 prostate cancer cells to verify the impact of BLM on DEPS. During the research, the phosphorylation response of histone proteins in PC tissues was measured using pathological scanning methods, and immunohistochemistry and automated western blot (WES) analyses were employed to validate these findings.
The authors studied whether BLM regulates the phosphorylation of a series of proto-oncogene signaling pathways to promote the progression of PC. It was found that silencing BLM in PC-3 cells significantly reduced their proliferative capacity. Furthermore, BLM downregulation significantly decreased the levels of phosphorylated protein kinase B (AKT (SR473)) and proline-rich AKT substrate of 40 kDa (PRAS40 (Thr246)), accompanied by an increase in reactive oxygen species (ROS) levels. Additionally, it was discovered that the inhibition of AKT and PRAS40 reduced BLM, but increased ROS levels, and induced apoptosis in PC cells[4].
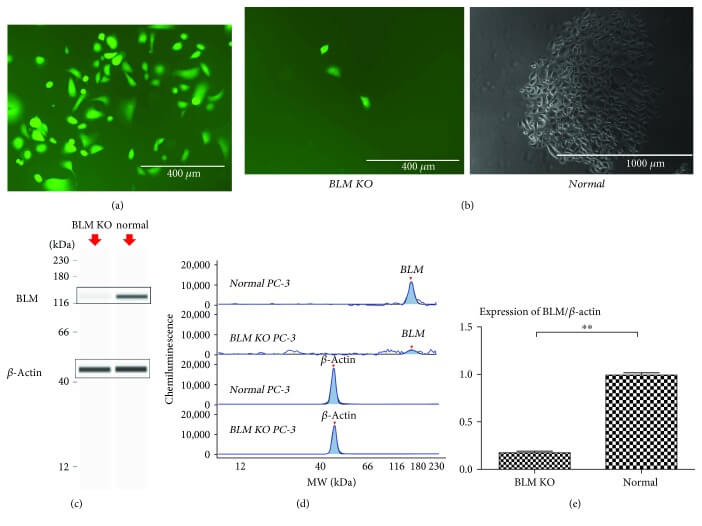
Figure 2: Targeted knockout of the BLM gene in PC-3 cells.
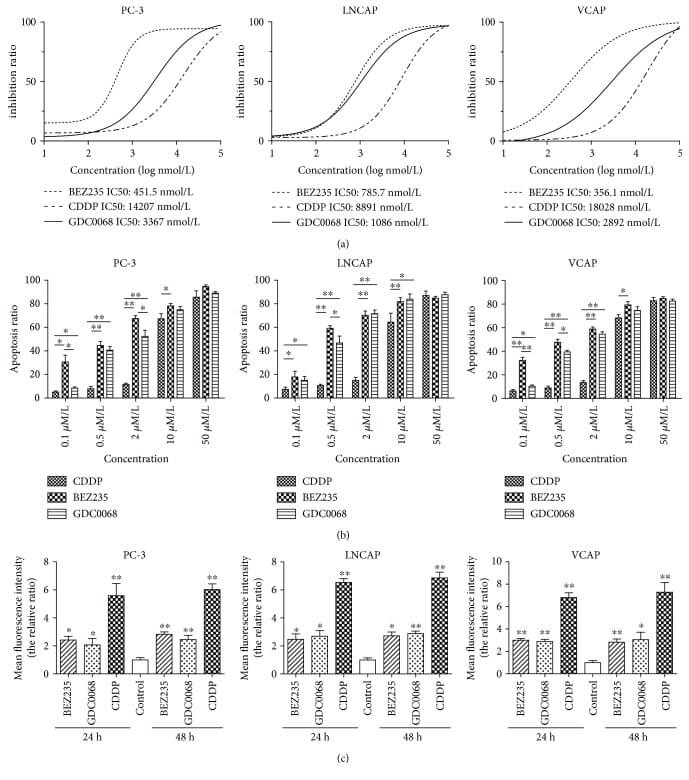
Figure 3: BEZ235 and GDC-0068 reduce proliferation and induce apoptosis in PC cells more effectively than that in CDDP.
Catalog# YC-C012
Origin: 22RV1 is a human prostate cancer epithelial cell line derived from a human prostate cancer xenograft (CWR22R). This xenograft originated from a castration-induced androgen-dependent CWR22 xenograft, which regressed after tumor development and then relapsed, continuously proliferating in nude mice.
Characteristics: This cell line represents a hyperdiploid stem cell line, evolved into a cytogenetic subline. Its basic karyotype is similar to that of the parental xenograft CWR22, and it is relatively simple, containing 50 chromosomes. It is tumorigenic in nude mice. The growth is weakly stimulated by dihydrotestosterone and shows an immune response with androgen receptor antibodies via Western blot. The growth is stimulated by epidermal growth factor but not inhibited by transforming growth factor β1. This cell line also expresses AR and PSA; cell growth is weakly activated by dihydrotestosterone and strongly activated by EGF, but it is not regulated by TGF-beta-1 inhibition; it has recently been found that the 22RV1 cell line can produce high-titer human XMRV retroviruses[5].
Application:
To investigate new mechanisms and potential targets for the treatment of castration-resistant prostate cancer (CRPC), a PRDX5 gene knockout cell line was constructed by Ubigene using 22RV1 cells, demonstrating that inhibiting PRDX5 can suppress DTP cell proliferation in culture, slow CRPC progression in animal models, and stabilize PSA progression and metastatic lesions in patients[6]. For more details, refer to Ubigene’s PRDX5 Knockout and Overexpression Cells Aid in Discovering New Mechanisms in Castration-Resistant Prostate Cancer Progression
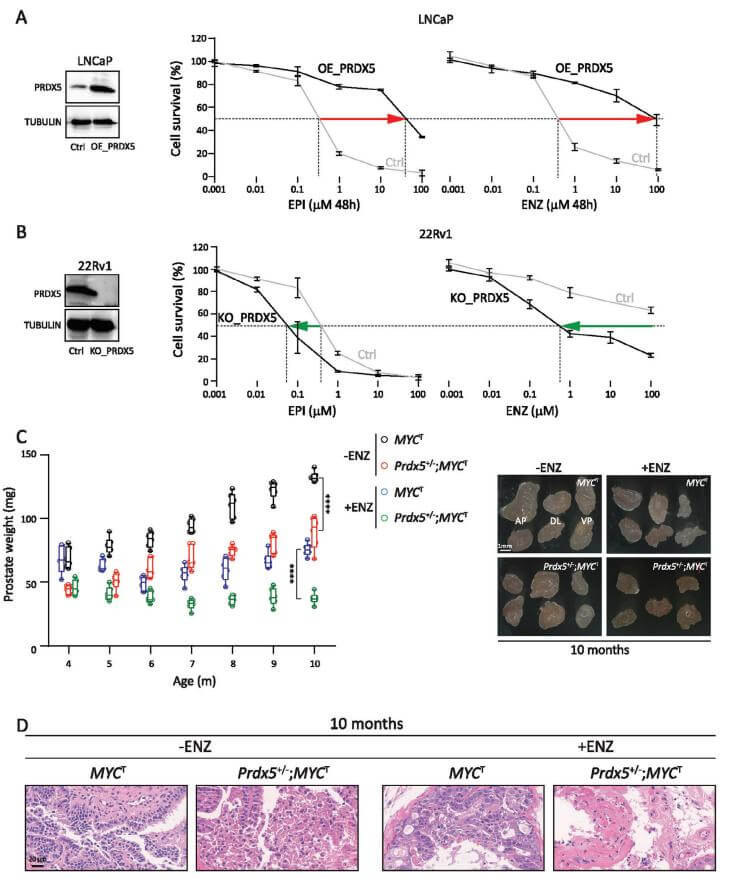
Figure 4: The Key Role of PRDX5 in AR Inhibitor Resistance and CRPC Progression
Based on the above cases, researches on prostate cancer primarily focus on discovering new treatment measures, while the application of gene editing technology and large-scale screening accelerates the progress of prostate cancer treatment research. Additionally, some studies may use multiple prostate cancer cell lines for cross-validation to enhance the reliability of research results. Researchers can select the most suitable cell lines according to your actual research goals and experimental conditions
Ubigene can provide gene editing (KO/KI/PM) and stable cell line customization services related to prostate cancer research. Feel free to inquire.
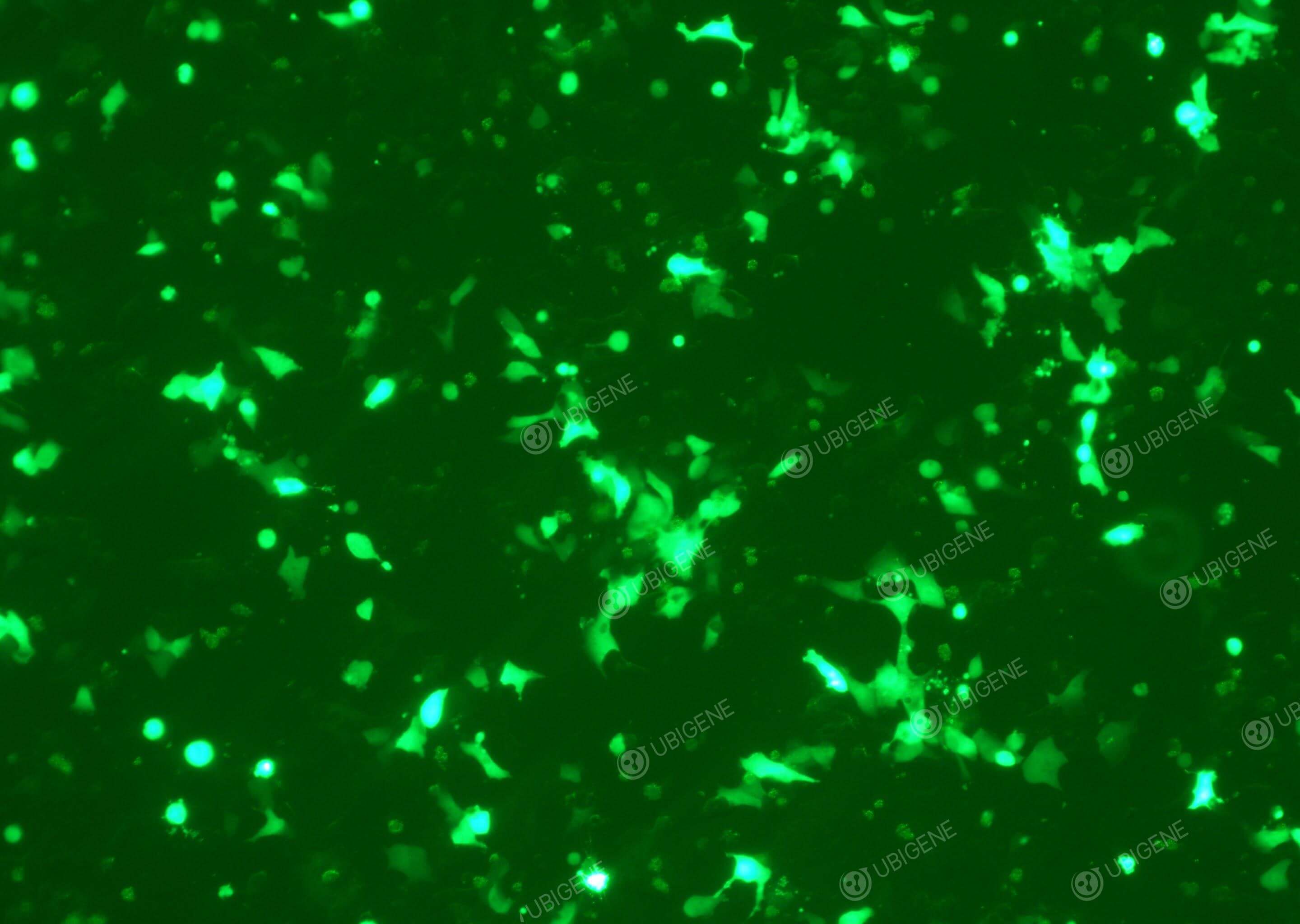
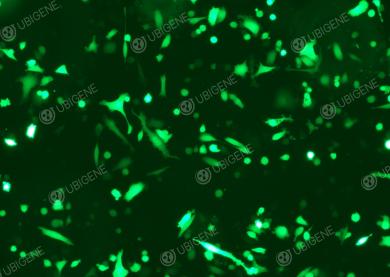
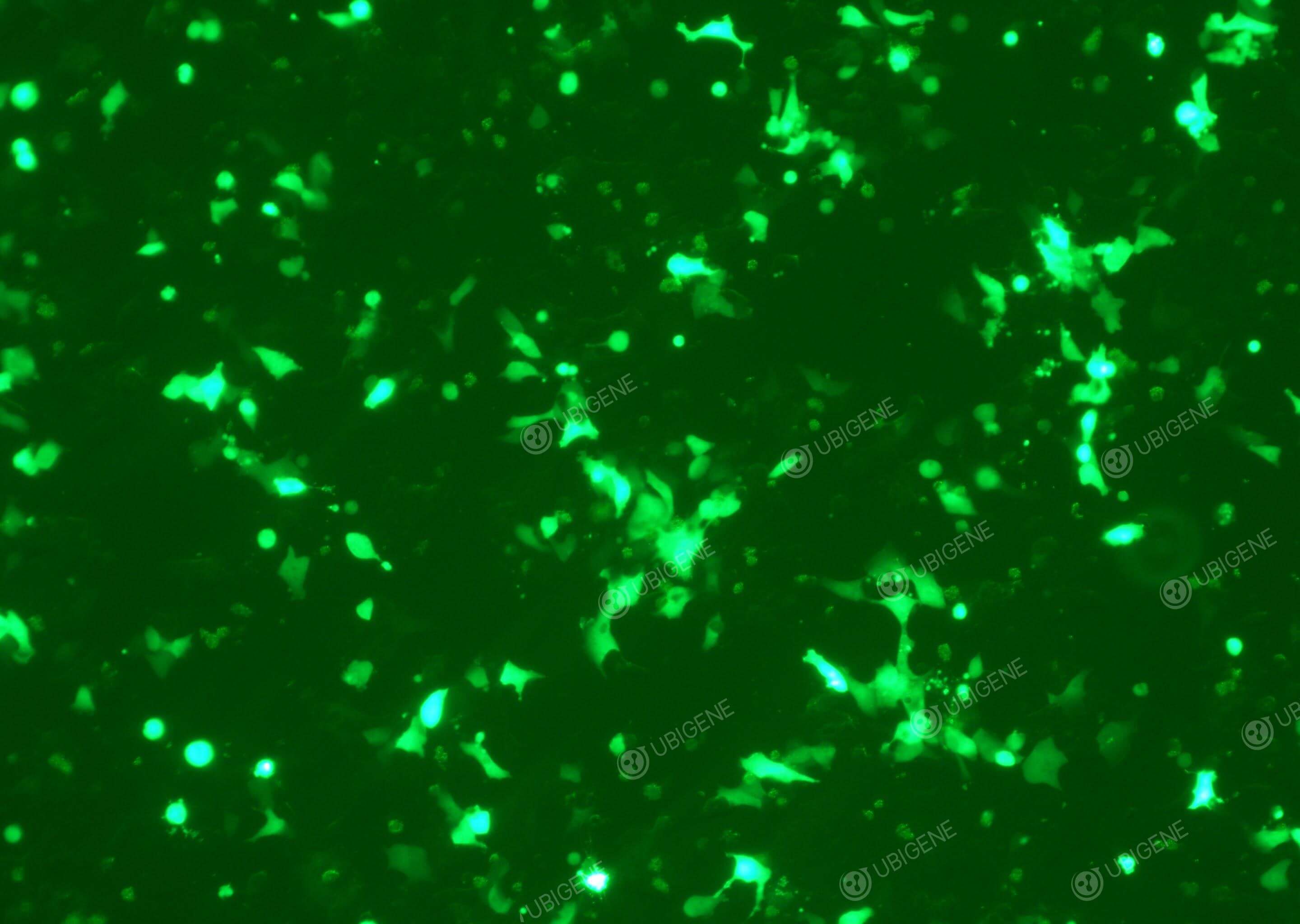
Gene editing experiment images (LNCaP Clone FGC - PC-3 - 22RV1)
References
[1] Juliana A Camargo et al. “The Effect of Gene Editing by CRISPR-Cas9 of miR-21 and the Indirect Target MMP9 in Metastatic Prostate Cancer.” Int J Mol Sci. 2023 Oct 3;24(19):14847. doi: 10.3390/ijms241914847.
[2] Sheng Tai et al. “PC3 Is a Cell Line Characteristic of Prostatic Small Cell Carcinoma.” Prostate: 2011 Mar 22;71(15):1668–1679. doi: 10.1002/pros.21383.
[3] Michael V Roes et al. "A Genome Wide CRISPR Screen Reveals That HOXA9 Promotes Enzalutamide Resistance in Prostate Cancer." Mol Cell Biol. 2024 Sep 20;44(12):529–542. doi: 10.1080/10985549.2024.2401465.
[4] Kun Chen et al. “Bloom Syndrome Protein Activates AKT and PRAS40 in Prostate Cancer Cells.” Oxid Med Cell Longev: 2019 May 9;2019:3685817. doi: 10.1155/2019/3685817
[5] R. Michael Sramkoski et al. "A new human prostate carcinoma cell line, 22Rv1." In Vitro Cell Dev Biol Anim. 1999 Jul-Aug;35(7):403-9. doi: 10.1007/s11626-999-0115-4.
[6] Wang, R., Mi, Y., Ni, J., Wang, Y., Ding, L., Ran, X., Sun, Q., Tan, S.Y., Koeffler, H.P., Feng, N., and Chen, Y.Q. (2023). Identification of PRDX5 as A Target for The Treatment of Castration-Resistant Prostate Cancer. Adv Sci (Weinh), e2304939. 10.1002/advs.202304939.








What are the commonly used cell lines for prostate cancer research?

Prostate cancer is one of the most common cancers in men, accounting for 26% of all diagnosed cases globally and 11% of estimated cancer-related deaths[1]. Currently, the standard treatment for prostate cancer is androgen receptor (AR) deprivation. However, when the disease progresses to the metastatic stage, it becomes ineffective due to androgen resistance, and acquired resistance remains a major issue. Prostate cancer cell models play an important role in prostate cancer research, which can be used to explore the pathogenesis, study cell biology characteristics, conduct drug screening and mechanism of action research, and discover new prostate cancer biomarkers. Next, let’s learn how three commonly used human prostate cancer cell lines provide strong support for prostate cancer research.
Catalog#: YC-C009
Characteristics: The cells exhibit slight adherent growth characteristics and are tumorigenic in nude mice. They respond to 5-alpha-dihydrotestosterone (growth regulators and acid phosphatase products) and express human prostate acid phosphatase, AR (androgen receptor, a marker for glandular formation and luminal differentiation), and PSA (prostate-specific antigen). Their growth is inhibited by androgen withdrawal, resembling human prostate adenocarcinoma. Neuroendocrine markers and stem cell-related marker CD44 are negative[2].
Application:
To identify the causes of enzalutamide sensitivity or resistance in prostate cancer treatment, the authors performed Genome-wide CRISPR knockout screen using LNCaP cells[1]. They discovered that key genes involved in epigenetic regulation and stem cell biology contribute to enzalutamide resistance. They also found that HOXA9 deletion increased enzalutamide sensitivity, while its overexpression caused resistance. Further experiments validated that HOXA9 inhibitors enhanced enzalutamide effectiveness and restored the response to enzalutamide in RB-p53 double-mutant cells. These findings suggest that inhibiting HOXA9 could be a therapeutic strategy for treating enzalutamide-resistant prostate cancer[3].

Figure 1: Genome-wide CRISPR knockout screening reveals that stem cell and epigenetic-related genes are crucial for cell viability under enzalutamide treatment
KO cells in stock - Prostate cancer cells and more KO cells, contact us!
Catalog#:YC-C010
Origin: Derived from a 62-year-old Caucasian male with stage IV prostate adenocarcinoma bone metastasis.
Characteristics: The cells exhibit low levels of acid phosphatase and 5-alpha-reductase activities, do not express AR (androgen receptor) or PSA (prostate-specific antigen), and their proliferation is independent of androgens, resembling small cell neuroendocrine carcinoma (SCNC) of the prostate. Neuroendocrine markers and stem cell-related marker CD44 are positive (+)[2].
Application:
To design new PC treatment interventions, CRISPR/Cas9-mediated homologous recombination gene editing was applied to perform site-specific elimination in PC-3 prostate cancer cells to verify the impact of BLM on DEPS. During the research, the phosphorylation response of histone proteins in PC tissues was measured using pathological scanning methods, and immunohistochemistry and automated western blot (WES) analyses were employed to validate these findings.
The authors studied whether BLM regulates the phosphorylation of a series of proto-oncogene signaling pathways to promote the progression of PC. It was found that silencing BLM in PC-3 cells significantly reduced their proliferative capacity. Furthermore, BLM downregulation significantly decreased the levels of phosphorylated protein kinase B (AKT (SR473)) and proline-rich AKT substrate of 40 kDa (PRAS40 (Thr246)), accompanied by an increase in reactive oxygen species (ROS) levels. Additionally, it was discovered that the inhibition of AKT and PRAS40 reduced BLM, but increased ROS levels, and induced apoptosis in PC cells[4].

Figure 2: Targeted knockout of the BLM gene in PC-3 cells.

Figure 3: BEZ235 and GDC-0068 reduce proliferation and induce apoptosis in PC cells more effectively than that in CDDP.
Catalog# YC-C012
Origin: 22RV1 is a human prostate cancer epithelial cell line derived from a human prostate cancer xenograft (CWR22R). This xenograft originated from a castration-induced androgen-dependent CWR22 xenograft, which regressed after tumor development and then relapsed, continuously proliferating in nude mice.
Characteristics: This cell line represents a hyperdiploid stem cell line, evolved into a cytogenetic subline. Its basic karyotype is similar to that of the parental xenograft CWR22, and it is relatively simple, containing 50 chromosomes. It is tumorigenic in nude mice. The growth is weakly stimulated by dihydrotestosterone and shows an immune response with androgen receptor antibodies via Western blot. The growth is stimulated by epidermal growth factor but not inhibited by transforming growth factor β1. This cell line also expresses AR and PSA; cell growth is weakly activated by dihydrotestosterone and strongly activated by EGF, but it is not regulated by TGF-beta-1 inhibition; it has recently been found that the 22RV1 cell line can produce high-titer human XMRV retroviruses[5].
Application:
To investigate new mechanisms and potential targets for the treatment of castration-resistant prostate cancer (CRPC), a PRDX5 gene knockout cell line was constructed by Ubigene using 22RV1 cells, demonstrating that inhibiting PRDX5 can suppress DTP cell proliferation in culture, slow CRPC progression in animal models, and stabilize PSA progression and metastatic lesions in patients[6]. For more details, refer to Ubigene’s PRDX5 Knockout and Overexpression Cells Aid in Discovering New Mechanisms in Castration-Resistant Prostate Cancer Progression

Figure 4: The Key Role of PRDX5 in AR Inhibitor Resistance and CRPC Progression
Based on the above cases, researches on prostate cancer primarily focus on discovering new treatment measures, while the application of gene editing technology and large-scale screening accelerates the progress of prostate cancer treatment research. Additionally, some studies may use multiple prostate cancer cell lines for cross-validation to enhance the reliability of research results. Researchers can select the most suitable cell lines according to your actual research goals and experimental conditions
Ubigene can provide gene editing (KO/KI/PM) and stable cell line customization services related to prostate cancer research. Feel free to inquire.



Gene editing experiment images (LNCaP Clone FGC - PC-3 - 22RV1)
References
[1] Juliana A Camargo et al. “The Effect of Gene Editing by CRISPR-Cas9 of miR-21 and the Indirect Target MMP9 in Metastatic Prostate Cancer.” Int J Mol Sci. 2023 Oct 3;24(19):14847. doi: 10.3390/ijms241914847.
[2] Sheng Tai et al. “PC3 Is a Cell Line Characteristic of Prostatic Small Cell Carcinoma.” Prostate: 2011 Mar 22;71(15):1668–1679. doi: 10.1002/pros.21383.
[3] Michael V Roes et al. "A Genome Wide CRISPR Screen Reveals That HOXA9 Promotes Enzalutamide Resistance in Prostate Cancer." Mol Cell Biol. 2024 Sep 20;44(12):529–542. doi: 10.1080/10985549.2024.2401465.
[4] Kun Chen et al. “Bloom Syndrome Protein Activates AKT and PRAS40 in Prostate Cancer Cells.” Oxid Med Cell Longev: 2019 May 9;2019:3685817. doi: 10.1155/2019/3685817
[5] R. Michael Sramkoski et al. "A new human prostate carcinoma cell line, 22Rv1." In Vitro Cell Dev Biol Anim. 1999 Jul-Aug;35(7):403-9. doi: 10.1007/s11626-999-0115-4.
[6] Wang, R., Mi, Y., Ni, J., Wang, Y., Ding, L., Ran, X., Sun, Q., Tan, S.Y., Koeffler, H.P., Feng, N., and Chen, Y.Q. (2023). Identification of PRDX5 as A Target for The Treatment of Castration-Resistant Prostate Cancer. Adv Sci (Weinh), e2304939. 10.1002/advs.202304939.







