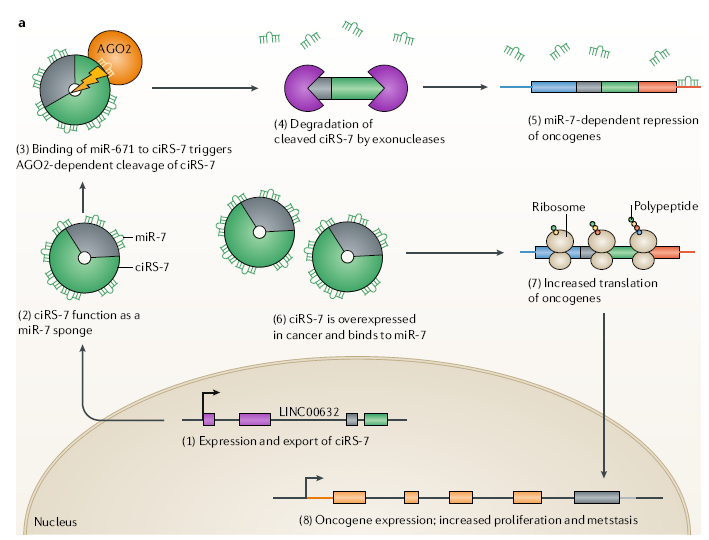
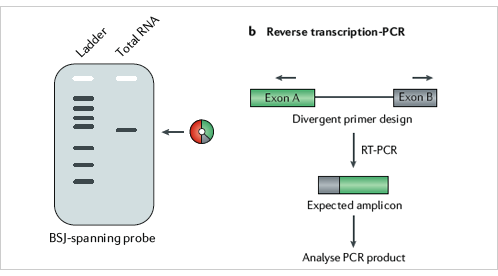
At present, the most studied is the relationship between circRNAs and tumors.
Some circRNAs promote tumor formation, such as circPvt1 in squamous cell
carcinomas of the head and neck, cirs-7 (CDr1as) in colorectal cancer,
esophageal squamous cell carcinoma and hepatocellular carcinoma. Some circRNAs
suppress tumors, such as circsMARCA5 and circ-SHPRH in glioblastoma. Some
circRNAs may play different roles in different tissues or cells, such as
circHiPK3, which is a proto-oncogene in rectal cancer, but suppresses cancer
cells in bladder cancer.
In addition to cancers, circRNA has been found to be closely related to
diabetes, cardiovascular disease, chronic inflammation and nervous system
diseases. It is believed that with the development of biotechnology and more
in-depth researches on circRNA, the formations and mechanisms of circRNAs can be
identified. circRNAs can play important roles in disease prevention, diagnosis
and treatment discovery.
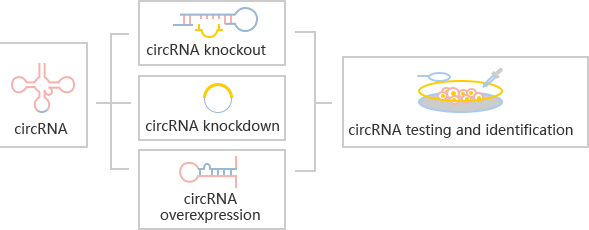
circRNA knockout refers to editing at the level of DNA to achieve the purpose of
a complete knockout. gRNA and Cas9 would be transferred into cells by virus
transduction or nucleofection. After drug screening, single clones would be
generated. Positive clones would be validated by sequencing.
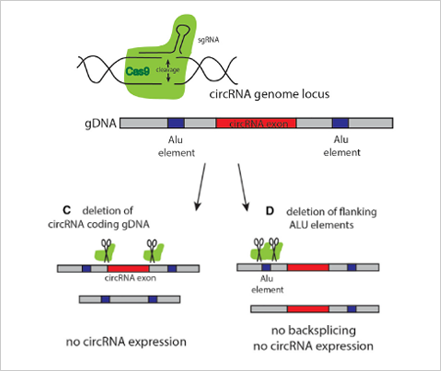
The most common strategies for circRNA knockout:
Strategy 1
The most commonly used method is to design two gRNAs at both ends of the
circRNA exon to knockout the whole cyclized exon sequence. Although this
strategy can knockout the circRNA, it will also affect the parent gene
encoding the protein, and the study on its function is not ideal.
Strategy 2
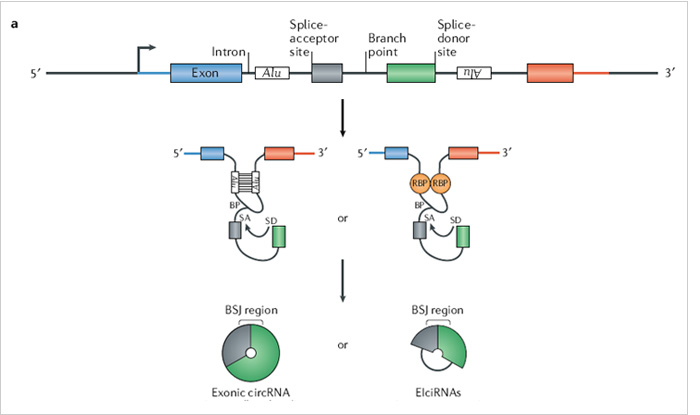
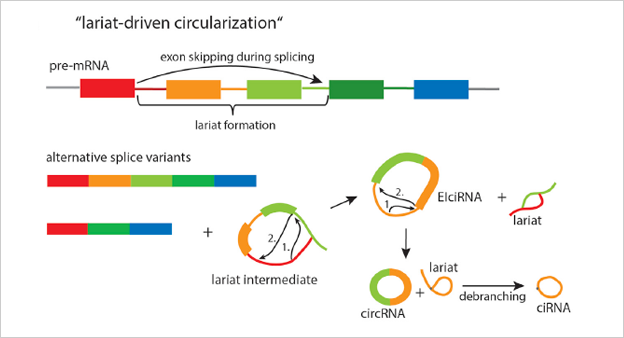
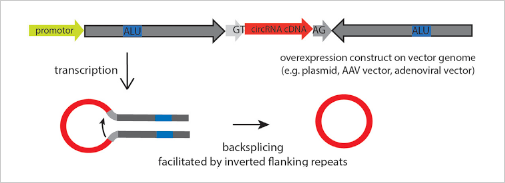
The ideal method is to knockout the loop forming elements (Alu) in the
flanking intron of the exon, so as to destroy the circRNA loop forming
without affecting the expression of the coding gene.(Fig 4.
Ubigene is experienced in designing strategy of knockout the
loop forming elements in the flanking intron of circRNA exon, to achieve the
purpose of knockout circRNA without affecting the expression of coding gene.
Combined with CRISPR-U™ technology, the positive clones of circRNA knockout can
be generated 10x faster than other common methods.
Case study:
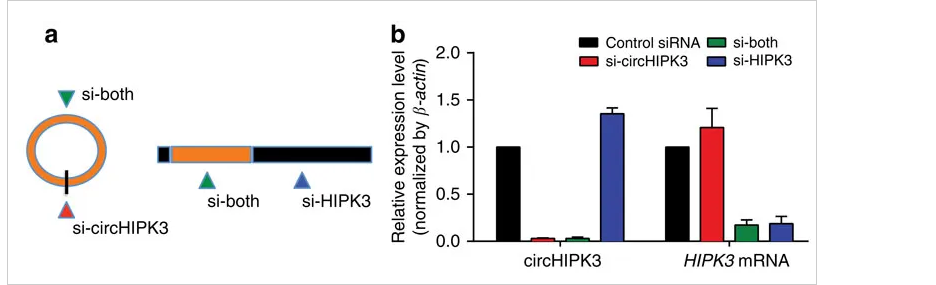
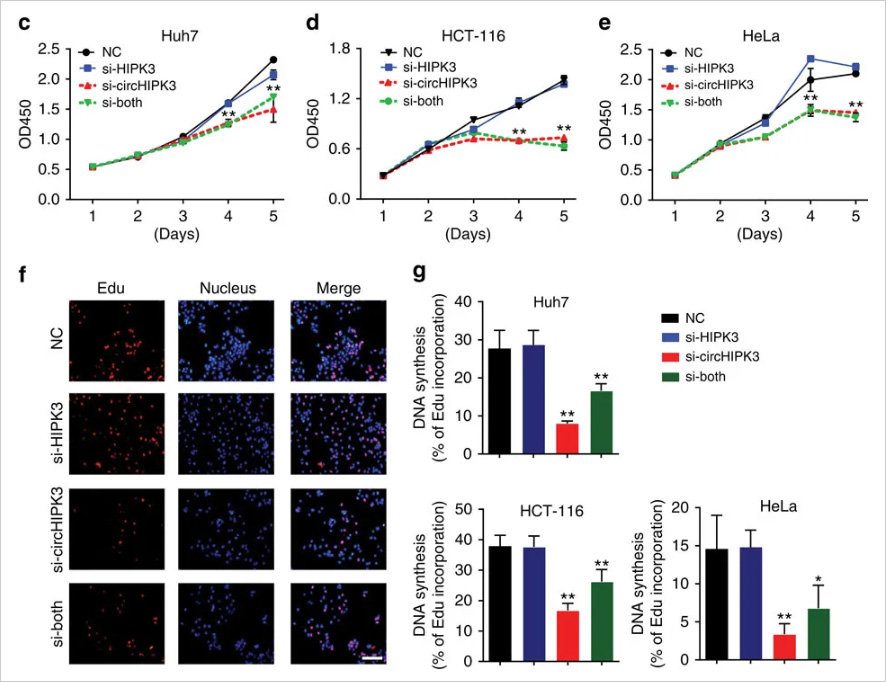
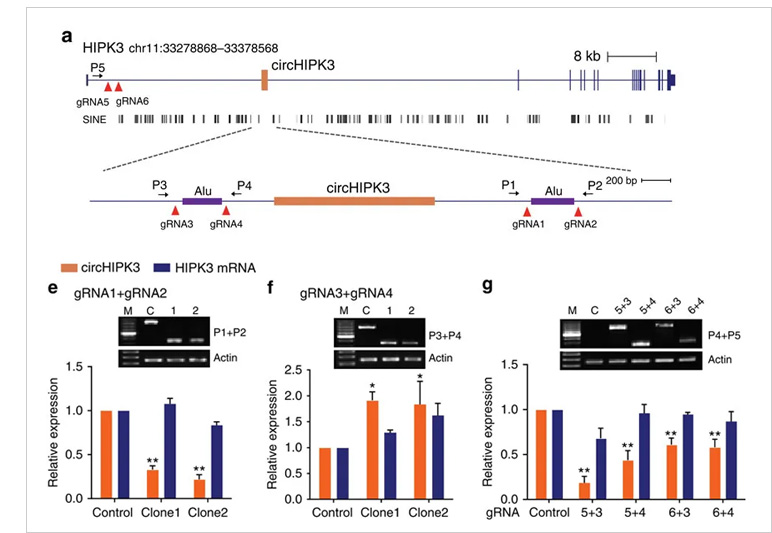
circ-HIPK3 is a kind of circRNA rich in human cells, which can combine with a
variety of miRNAs as a regulator of cell growth and affect the formation of
tumors. In order to verify how circ-HIPK3 forms into circle, it is necessary to find the
loop forming elements in flanking intron. A pair of sgRNA is designed for the two
Alu elements predicted at upstream and downstream respectively. The predicted loop
forming elements are knockout by CRISPR/Cas9 system to detect whether the expression
of circRNA changes. After PCR and RT-qPCR verification, it was found that the
expression of circ-HIPK3 was significantly down-regulated after knockout of
downstream loop forming elements, while the expression of circ-HIPK3 was not
decreased but increased after knockout of upstream loop forming elements. It was
speculated that there were too many loop forming elements in the upstream and the
prediction was not accurate. In order to further verify the RNA circulation driven
by other elements, the large fragment of intron in the upstream of the element was
knockout by co-injection of gRNA3 or gRNA4 with gRNA5 or gRNA6. RT-qPCR results
showed that the expression of circ-HIPK3 decreased, indicating that other loop
forming elements of circ-HIPK3 exist.
Reference:
Zheng, Q., Bao, C., Guo, W., Li, S., Chen, J., Chen, B., ... & Liang, L. (2016).
Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by
sponging multiple miRNAs. Nature communications, 7(1), 1-13.