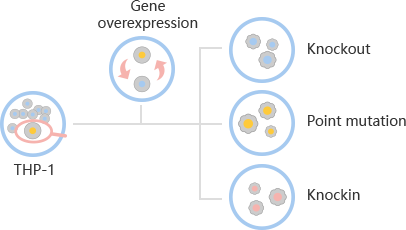
A human leukemic cell line (THP-1) cultured from the blood of a boy with acute monocytic leukemia. Since its establishment in 1980, THP-1 cells have been widely used in the research
of monocyte and macrophage related mechanisms, signaling pathways, nutrient and drug
transportation. The morphological and functional characteristics of THP-1 are very similar
to human primary monocytes (including cell differentiation markers). Compared with human
peripheral blood monocytes (PBMC), THP-1 is easier to be cultured and has a more consistent
background. Therefore, THP-1 is a commonly used acute monocytic leukemia cell line in
various laboratories, and it is an ideal tool for studying immunity and inflammation.
Application of THP-1: macrophage and inflammation model
THP-1 can be differentiated into M1/M2 macrophages and release
corresponding cytokines.
· M1 macrophage
polarization:
THP-1 can be induced to differentiate into macrophages by Phorbol 12-myristate 13-acetate
(PMA), and then M1 polarization can be induced by lipopolysaccharide (LPS) and IFN -γ,
releasing TNF -α, IL-6 and other cytokines. This is a typical inflammatory model.
· M2 macrophage
polarization:
M2 polarization can be induced by IL-4, IL-13 and
macrophage colony-stimulating factor (M-CSF). TGF - β, IL-10 and other inhibitory cytokines
can be released. This is similar to the process of tissue repair and reconstruction in the
late stage of inflammation.
· Atherosclerotic
inflammation model:
Under the action of oxidized low-density lipoprotein
(ox-LDL), macrophages can further become foam cells. This is a pathological cell in
atherosclerotic plaques and is a chronic inflammation model.
CRISPR-U™ for efficient gene editing in THP-1 cells
THP-1 is a near-tetraploid suspension cell, and the success rate of THP-1 is very low by
conventional gene-editing methods.
CRISPR/Cas9 is widely used to construct gene-editing
THP-1 model because of its simple, high efficiency and low toxicity.
CRISPR-U™, developed by
Ubigene, is more efficient than general CRISPR/Cas9 in double-strand breaking, and CRISPR-U™
can greatly improve the efficiency of homologous recombination, easily achieve
knockout
(KO), point mutation (PM) and
knockin (KI).
Ubigene can customize the gene-editing THP-1 cell line and other monocytes that you are
interested in, as well as generate various genes overexpression in THP-1 cell line. THP-1 Cell Line Gene-editing Services:
Technical advantages

Exclusive innovation, 10 times more efficient in gene-editing.

Successfully edit genes on more than 100 types of cell lines.

Easily generate knockout (KO), point mutation (PM) and knockin (KI) in vitro and in
vivo.

CRISPR-U™ offers a 100% mutation guarantee. No mutation, no charge!
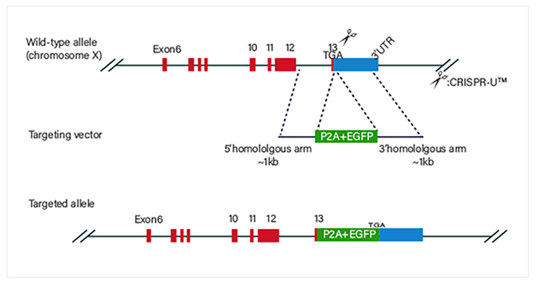
CRISPR-U™ gene knockout THP-1 cell line: gRNA and Cas9 are
transferred into THP-1 cells by nucleofection. After drug screening, single
clones would be generated. Positive clones would be validated by sequencing.
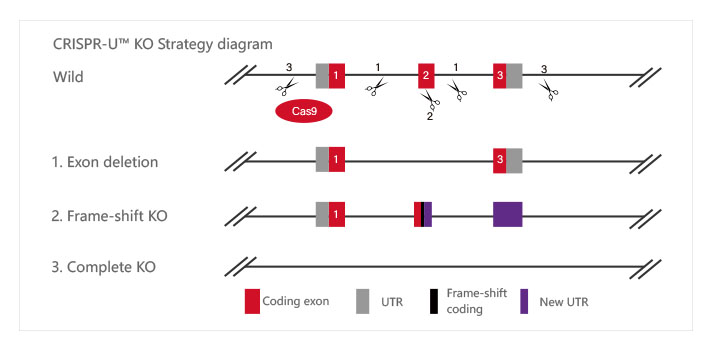
Knockout Strategies:
| Type | Strategy | Application |
| Short fragment removal | Guide RNAs target introns at both sides of exon 2 and the number of bases in exon 2 is not a multiple of 3, which can cause frame-shift mutation. | Study of gene function through gene defect |
| Frame-shift mutation | Guide RNA targets the exon, and the base number of deletion is not a multiple of 3. After knockout, frame-shift mutation would cause gene knockout. |
| Large fragment removal | Complete removal of the coding sequence to achieve gene knockout. |
Case Study:
Key genes for macrophages clearing pathogens was found
by CRISPR/Cas9 mediated gene knock-out THP-1 model
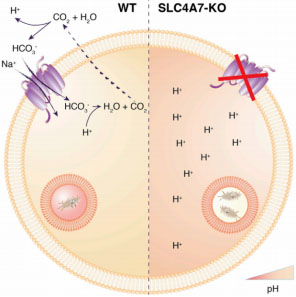
Phagosome acidification of macrophages is an essential step to eliminate
pathogens. Phagosome acidification is closely related to the metabolism of
macrophages and the transportation of nutrients. And the transportation of
metabolites is closely related to solute carrier (SLC) protein. The researchers
found that the bicarbonate transporter SLC4A7 in the SLC family is an essential
gene for phagosome acidification of macrophages. In CRISPR/Cas9 mediated SLC4A7
knockout THP-1 cell line, the ability of phagosome acidification and killing
bacteria was reduced. The acidity of the phagocyte was increased after the
supplementation of SLC4A7. This indicates that SLC4A7 mediated bicarbonate driven in macrophages is
essential for the maintenance of cytoplasmic pH and phagosome
acidification.
Reference:
Sedlyarov V, Eichner R, Girardi E, et al. The bicarbonate transporter SLC4A7
plays a key role in macrophage phagosome acidification[J]. Cell host & microbe,
2018, 23(6): 766-774. e5.
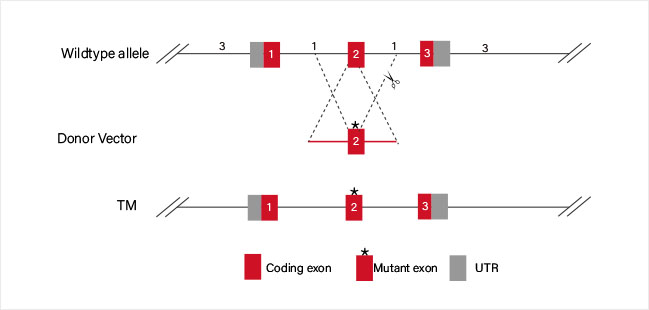
CRISPR-U™ Point Mutation THP-1 Cell Line: THP-1 cell line would be efficiently co-transfected with gRNA, Cas9 and ssODN.
After drug screening, single clones would be generated. Positive clones would be
validated by sequencing.
· Disease model
generation
ssODN carrying point mutaion which replaces the WT sequence
by HDR.
· Disease model rescuing
ssODN carrying WT sequence which replaces the mutated site
by HDR.
Case Study:
CRISPR/Cas9 mediated Chronic granulomatous disease
(CGD) THP-1 cell line model is helpful to develop better disease treatments
Chronic granulomatous disease (CGD) is a rare X-linked genetic disease. Due to
the mutation or deficient of CYBB gene, macrophages lack nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase, and cannot produce hydrogen peroxide to
effectively kill the invading microorganisms. This usually leads to serious
repeated infections caused by bacteria, fungi and other microorganisms. Some
researchers used CRISPR/Cas9 technology to knockout CYBB gene and generate point
mutation c.90c>G (found in a CGD patient) in THP-1 cells, and successfully
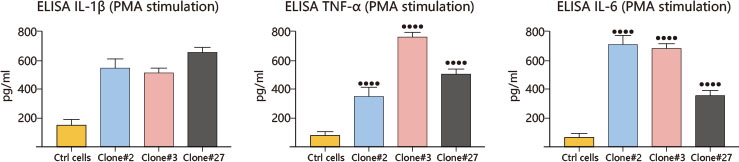
constructed the CGD model. Compared with wild-type THP-1 cells, two KO clones
(#3 and #27) and a point mutation clone (#2, c.90c > G) showed decrease in H2O2
level after PMA and LPS induction, and a significant increase in IL-1β, TNF-α
and IL-6 release, which was consistent with the behavior of macrophages in
CGD.This CGD model provides a powerful tool for disease study and will help
to develop better treatments.
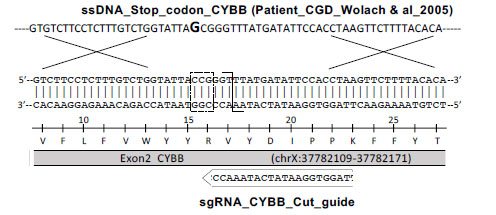
Compared with wild-type THP-1 cells, two KO clones (#3 and
#27) and a point mutation clone (#2, c.90c > G) showed decrease in H2O2 level
after PMA and LPS induction, and a significant increase in IL-1β, TNF-α and IL-6
release, which was consistent with the behavior of macrophages in CGD. This CGD
model provides a powerful tool for disease study and will help to develop better
treatments.
Reference:
Benyoucef A, Marchitto L, Touzot F. CRISPR gene-engineered CYBBko THP-1 cell
lines highlight the crucial role of NADPH-induced reactive oxygen species for
regulating inflammasome activation[J]. Journal of Allergy and Clinical
Immunology, 2020.
CRISPR-U™ Gene Knockin THP-1 Cell Line: THP-1 cell line would be co-transfected with gRNA, Cas9 and donor vector. After
drug screening, single clones would be generated. Positive clones would be
validated by sequencing.
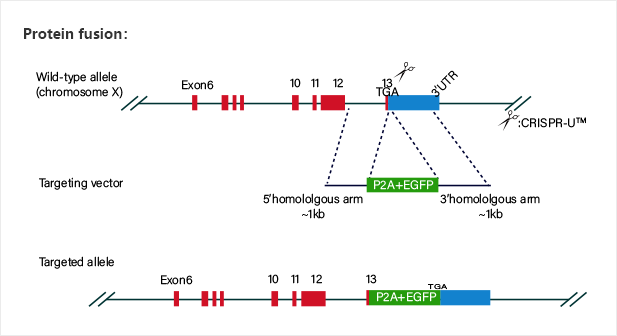
Gene knockin at Safe harbors such as hROSA26 and AAVS1 not
only avoids random insertion in genome, but also achieves overexpression of
target gene.
Case Study:
The signal pathway of intracellular antiviral response was
confirmed by the THP-1 Cell Models of gene knock-out and knock-in.
The abnormal location of DNA in the cytoplasm is usually related to virus
infection or tumor. The cGAS-cGAMP-STING pathway can detect the existence of
cytosolic dsDNA, and induce a strong immune response, producing interferon and
activating other immune response genes. RIG1-MAVS can detect pppRNA (dsRNA, the
genome of some viruses) in cytoplasm and induce immune response. Sometimes there
is a complex of RNA and DNA in the cytoplasm, which usually occurs in the case
of some virus infection. In order to study which pathway that the RNA-DNA
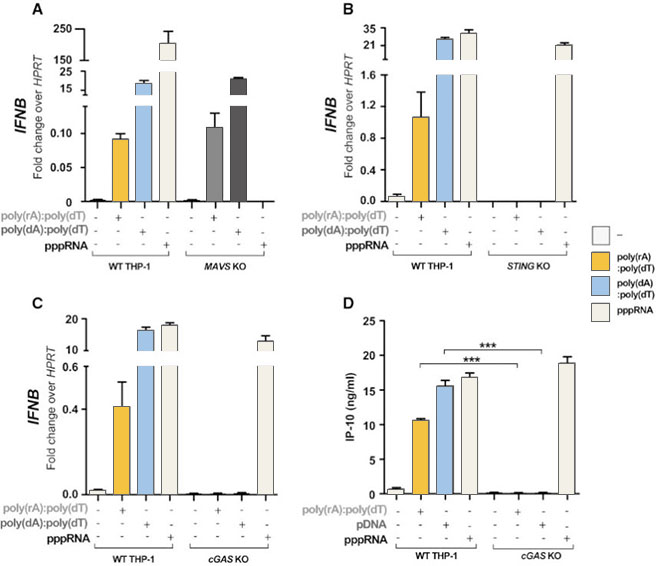
complex activates the immune response, the researchers generated MAVS, cGAS,
STING knockout THP-1 cell lines, and introduced dsDNA, ppRNA and RNA-DNA complex
into the cells respectively. It was found that the RNA-DNA complex is activated
by the cGAS-cGAMP-STING pathway.
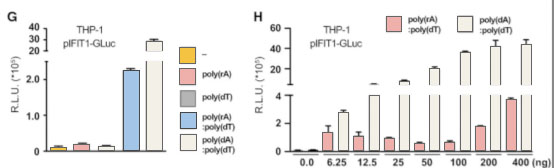
Then, the researchers used CRISPR/Cas9 technology to insert
2A-GLuc into the IFIT1 gene. IFIT1 is a typical interferon activated gene.
Subsequent experiments showed that after the introduction of RNA-DNA complex,
the expression of Gluc was driven by the activation of IFIT1 promoter due to the
expression of interferon. These results further proved that the RNA-DNA complex
in the cytoplasm activated the immune response through the cGAS-cGAMP-STING
pathway.
Reference:
Mankan A K, Schmidt T, Chauhan D, et al. Cytosolic RNA: DNA hybrids activate the
cGAS–STING axis[J]. The EMBO journal, 2014, 33(24): 2937-2946.