Location:Home > Application > Luciferase Labeled Tumor Cell Lines in Tumor Research
Expert Insights | Luciferase Labeled Tumor Cell Lines in Tumor Research
Tumor research is a crucial branch in the biomedical field, and mouse tumor models provide a powerful tool for studying tumor growth, metastasis, and drug efficacy. However, traditional methods of tumor monitoring often require sacrificing experimental animals, which not only increases research costs but may also impact the accuracy of experimental results.
luciferase labeled tumor cell lines have brought innovation to tumor research. These cells stably express luciferase, allowing real-time tracking of tumor progression through bioluminescence imaging. This non-invasive monitoring method reduces the need for animals and enables continuous monitoring of tumor growth dynamics. This article will explore the application and operation of luciferase expressing tumor cell lines.
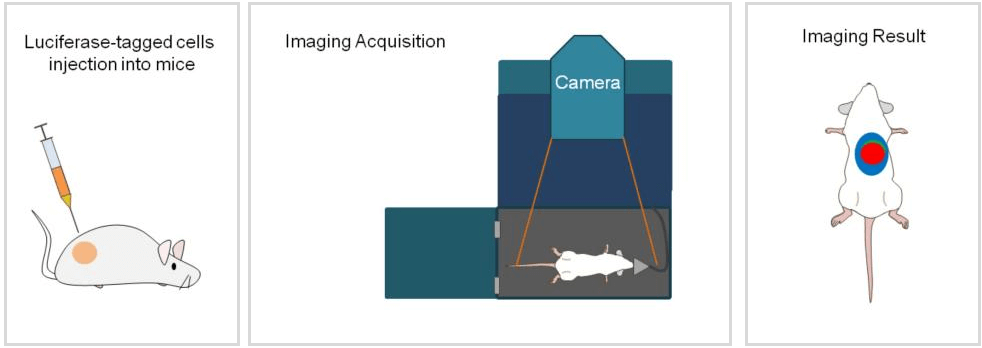
Figure 1 Schematic diagram of the application process of Luciferase stable cells in in vivo
Before starting the experiment, it is necessary to prepare the cells and animals. But what kind of luciferase cells should be selected? Here is some information for your reference:
Human-derived tumor cell lines: Suitable for studying the mechanisms of human cancer and evaluating anti-cancer drug efficacy.
Mouse-derived tumor cell lines: Suitable for studying the interactions between tumor cells and the host immune system, as well as the effects of the immune system on tumor growth and metastasis.
Immune-deficient mouse models: Human-derived tumor cell lines are typically used in immune-deficient mouse models, such as nude mice or severe combined immunodeficiency (SCID) mice, to avoid immune rejection.
Immune-intact mouse models: Mouse-derived tumor cell lines can be transplanted into mice with the same genetic background that do not experience immune rejection.
Tumor type: Select the appropriate cell line based on the type of tumor being studied.
Metastatic characteristics: If studying metastasis, choose cell lines known to have metastatic capabilities.
Tumorigenicity: Select cell lines with good tumorigenicity in animal models to ensure reliable experimental results.
Luciferase expression: Choose cell lines with high luciferase activity to ensure clear signals in live imaging.
Cell culture conditions: Select cell lines that are easy to operate and culture.
Cell passages: Avoid using cell lines with excessively high passages.
Cell condition: Choose cell lines with good condition and no contamination.
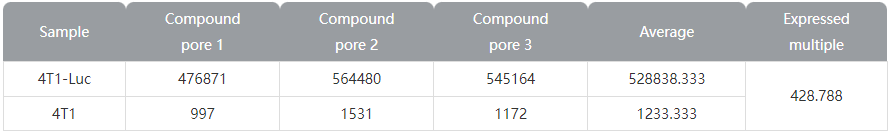
Figure 2 Luciferase expression results of Ubigene's 4T1-Luc cells
According to some references and other sources, choose widely used cell lines with abundant data for easy comparison and validation.
After selecting the appropriate cell line and mouse model, the next step is the specific operational procedures for mouse tumor experiments.
1.1. Generation of luciferase-expressing tumor cell lines
Culture Luciferase stable cells under suitable conditions in the culture medium, ensuring the cells are in good condition. When the cells reach 80-90% confluence, digest the cells with trypsin, centrifuge and resuspend the cells in PBS or serum-free medium, count the cells, adjust the concentration to 5x10^6 cells/100 µL (varying depending on the cell line), and keep on ice.
1.2. Mouse Preparation
Select the mouse strain: Choose an appropriate immune-deficient mouse (such as nude mice, SCID) or an immune-intact mouse based on experimental requirements.
Acclimatization: Acclimate the mice to the laboratory environment before the experiment.
Anesthetize the mouse, shave the injection site, disinfect with alcohol, aspirate the cell suspension using a 1 mL syringe, and inject the suspension subcutaneously. Besides subcutaneous injection, other methods include orthotopic injection, intraperitoneal injection, intravenous injection, etc., chosen based on the research purpose.
Tips:
Heterotopic transplantation involves injecting tumor cells directly into a non-primary site in the experimental animal, most commonly subcutaneously. This method is simple, facilitates tumor establishment and monitoring, but doesn't completely mimic the natural tumor growth environment and microenvironment.
Orthotopic transplantation involves injecting tumor cells into the site where tumors naturally occur, such as the liver, lungs, mammary glands, etc. This method closely resembles natural tumor growth conditions and better simulates the interaction between the tumor and the host.
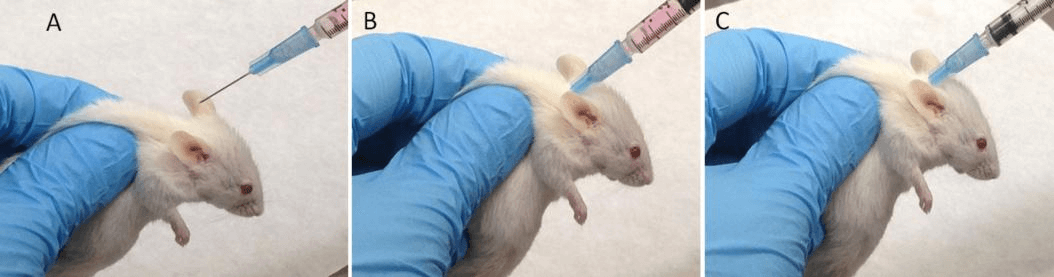
Figure 3 Subcutaneous injection of cells in mice
Inject luciferase substrate intraperitoneally or intravenously, let the mouse move freely for 6-8 minutes, then anesthetize the mouse. 15-20 minutes post-infection, capture and record the bioluminescent signal using a BLI device.
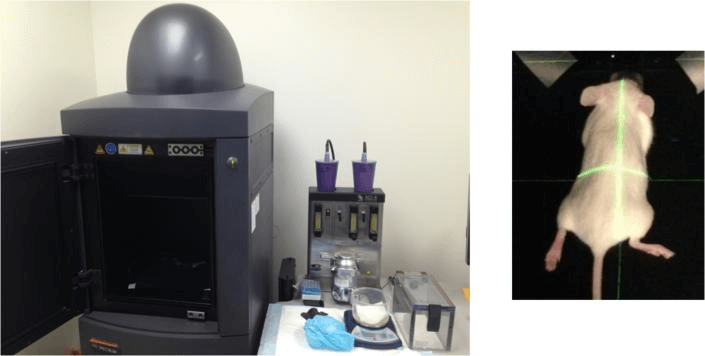
Figure 4 BLI device and the mouse placed in camera box
Ubigene's Luciferase Cell Bank offers 200+ stable luciferase-expressing cell lines, known for strong specificity, high sensitivity, high imaging quality, and precise quantification of luminescent intensity. These cells effectively assist in transcription factor regulation studies, live animal imaging, cell tracking, and other experiments.
Advantages of Ubigene's Luciferase Cells
|
STR authenticated✔ Low passage, high activity✔ |
Luciferase activity detected✔ High-impact articles cited✔ |
After-sales procedure✔ Technical support✔ |
Example Results of Using Ubigene's Luciferase Cells for Tumor Formation
|
Partial published articles using Ubigene's Luciferase Cells
| Articles | Published in | IF |
| Adaptive Design of Nanovesicles Overcoming Immunotherapeutic Limitations of Chemotherapeutic Drugs through Poliovirus Receptor Blockade | ACS nano | 17.1 |
| Cell surface patching via CXCR4-targeted nanothreads for cancer metastasis inhibition | Nature Communications | 16.6 |
| Vitamin D binding protein (VDBP) hijacks twist1 to inhibit vasculogenic mimicry in hepatocellular carcinoma | Theranostics | 12.4 |
| Deterministic Single Cell Encapsulation in PEG Norbornene Microgels for Promoting anti‐Inflammatory Response and Therapeutic Delivery of Mesenchymal Stromal Cells | Advanced Healthcare Materials | 10.0 |
Conclusion
Ubigene's luciferase labeled tumor cell lines revolutionize in vivo tumor research, providing real-time bioluminescence imaging for precise tumor tracking. These luciferase expressing tumor cell lines are crucial for generating high-quality data in cancer research, as showcased in impactful publications. For advanced luciferase tumor imaging and generation of luciferase-expressing tumor cell lines, trust Ubigene's expertise and reliable cell lines.
References
1.Carceles-Cordon, M., Rodriguez-Fernandez, I., Rodriguez-Bravo, V., Cordon-Cardo, C. and Domingo-Domenech, J. (2016). In vivo Bioluminescence Imaging of Luciferase-labeled Cancer Cells. Bio-protocol 6(6): e1762.
2.Sinha, D., Pieterse, Z. and Kaur, P. (2018). Qualitative in vivo Bioluminescence Imaging. Bio-protocol 8(18): e3020. DOI: 10.21769/BioProtoc.3020
3.Wang C, Xie GM, Zhang LP, et al. High Engraftment and Metastatic Rates in Orthotopic Xenograft Models of Gastric Cancer via Direct Implantation of Tumor Cell Suspensions. Cancers (Basel). 2024;16(4):759.


Expert Insights | Luciferase Labeled Tumor Cell Lines in Tumor Research

Tumor research is a crucial branch in the biomedical field, and mouse tumor models provide a powerful tool for studying tumor growth, metastasis, and drug efficacy. However, traditional methods of tumor monitoring often require sacrificing experimental animals, which not only increases research costs but may also impact the accuracy of experimental results.
luciferase labeled tumor cell lines have brought innovation to tumor research. These cells stably express luciferase, allowing real-time tracking of tumor progression through bioluminescence imaging. This non-invasive monitoring method reduces the need for animals and enables continuous monitoring of tumor growth dynamics. This article will explore the application and operation of luciferase expressing tumor cell lines.

Figure 1 Schematic diagram of the application process of Luciferase stable cells in in vivo
Before starting the experiment, it is necessary to prepare the cells and animals. But what kind of luciferase cells should be selected? Here is some information for your reference:
Human-derived tumor cell lines: Suitable for studying the mechanisms of human cancer and evaluating anti-cancer drug efficacy.
Mouse-derived tumor cell lines: Suitable for studying the interactions between tumor cells and the host immune system, as well as the effects of the immune system on tumor growth and metastasis.
Immune-deficient mouse models: Human-derived tumor cell lines are typically used in immune-deficient mouse models, such as nude mice or severe combined immunodeficiency (SCID) mice, to avoid immune rejection.
Immune-intact mouse models: Mouse-derived tumor cell lines can be transplanted into mice with the same genetic background that do not experience immune rejection.
Tumor type: Select the appropriate cell line based on the type of tumor being studied.
Metastatic characteristics: If studying metastasis, choose cell lines known to have metastatic capabilities.
Tumorigenicity: Select cell lines with good tumorigenicity in animal models to ensure reliable experimental results.
Luciferase expression: Choose cell lines with high luciferase activity to ensure clear signals in live imaging.
Cell culture conditions: Select cell lines that are easy to operate and culture.
Cell passages: Avoid using cell lines with excessively high passages.
Cell condition: Choose cell lines with good condition and no contamination.

Figure 2 Luciferase expression results of Ubigene's 4T1-Luc cells
According to some references and other sources, choose widely used cell lines with abundant data for easy comparison and validation.
After selecting the appropriate cell line and mouse model, the next step is the specific operational procedures for mouse tumor experiments.
1.1. Generation of luciferase-expressing tumor cell lines
Culture Luciferase stable cells under suitable conditions in the culture medium, ensuring the cells are in good condition. When the cells reach 80-90% confluence, digest the cells with trypsin, centrifuge and resuspend the cells in PBS or serum-free medium, count the cells, adjust the concentration to 5x10^6 cells/100 µL (varying depending on the cell line), and keep on ice.
1.2. Mouse Preparation
Select the mouse strain: Choose an appropriate immune-deficient mouse (such as nude mice, SCID) or an immune-intact mouse based on experimental requirements.
Acclimatization: Acclimate the mice to the laboratory environment before the experiment.
Anesthetize the mouse, shave the injection site, disinfect with alcohol, aspirate the cell suspension using a 1 mL syringe, and inject the suspension subcutaneously. Besides subcutaneous injection, other methods include orthotopic injection, intraperitoneal injection, intravenous injection, etc., chosen based on the research purpose.
Tips:
Heterotopic transplantation involves injecting tumor cells directly into a non-primary site in the experimental animal, most commonly subcutaneously. This method is simple, facilitates tumor establishment and monitoring, but doesn't completely mimic the natural tumor growth environment and microenvironment.
Orthotopic transplantation involves injecting tumor cells into the site where tumors naturally occur, such as the liver, lungs, mammary glands, etc. This method closely resembles natural tumor growth conditions and better simulates the interaction between the tumor and the host.

Figure 3 Subcutaneous injection of cells in mice
Inject luciferase substrate intraperitoneally or intravenously, let the mouse move freely for 6-8 minutes, then anesthetize the mouse. 15-20 minutes post-infection, capture and record the bioluminescent signal using a BLI device.

Figure 4 BLI device and the mouse placed in camera box
Ubigene's Luciferase Cell Bank offers 200+ stable luciferase-expressing cell lines, known for strong specificity, high sensitivity, high imaging quality, and precise quantification of luminescent intensity. These cells effectively assist in transcription factor regulation studies, live animal imaging, cell tracking, and other experiments.
Advantages of Ubigene's Luciferase Cells
|
STR authenticated✔ Low passage, high activity✔ |
Luciferase activity detected✔ High-impact articles cited✔ |
After-sales procedure✔ Technical support✔ |
Example Results of Using Ubigene's Luciferase Cells for Tumor Formation
|
Partial published articles using Ubigene's Luciferase Cells
| Articles | Published in | IF |
| Adaptive Design of Nanovesicles Overcoming Immunotherapeutic Limitations of Chemotherapeutic Drugs through Poliovirus Receptor Blockade | ACS nano | 17.1 |
| Cell surface patching via CXCR4-targeted nanothreads for cancer metastasis inhibition | Nature Communications | 16.6 |
| Vitamin D binding protein (VDBP) hijacks twist1 to inhibit vasculogenic mimicry in hepatocellular carcinoma | Theranostics | 12.4 |
| Deterministic Single Cell Encapsulation in PEG Norbornene Microgels for Promoting anti‐Inflammatory Response and Therapeutic Delivery of Mesenchymal Stromal Cells | Advanced Healthcare Materials | 10.0 |
Conclusion
Ubigene's luciferase labeled tumor cell lines revolutionize in vivo tumor research, providing real-time bioluminescence imaging for precise tumor tracking. These luciferase expressing tumor cell lines are crucial for generating high-quality data in cancer research, as showcased in impactful publications. For advanced luciferase tumor imaging and generation of luciferase-expressing tumor cell lines, trust Ubigene's expertise and reliable cell lines.
References
1.Carceles-Cordon, M., Rodriguez-Fernandez, I., Rodriguez-Bravo, V., Cordon-Cardo, C. and Domingo-Domenech, J. (2016). In vivo Bioluminescence Imaging of Luciferase-labeled Cancer Cells. Bio-protocol 6(6): e1762.
2.Sinha, D., Pieterse, Z. and Kaur, P. (2018). Qualitative in vivo Bioluminescence Imaging. Bio-protocol 8(18): e3020. DOI: 10.21769/BioProtoc.3020
3.Wang C, Xie GM, Zhang LP, et al. High Engraftment and Metastatic Rates in Orthotopic Xenograft Models of Gastric Cancer via Direct Implantation of Tumor Cell Suspensions. Cancers (Basel). 2024;16(4):759.


